FDA affirms safety of diabetes drug Avandia, lifts restrictions on use
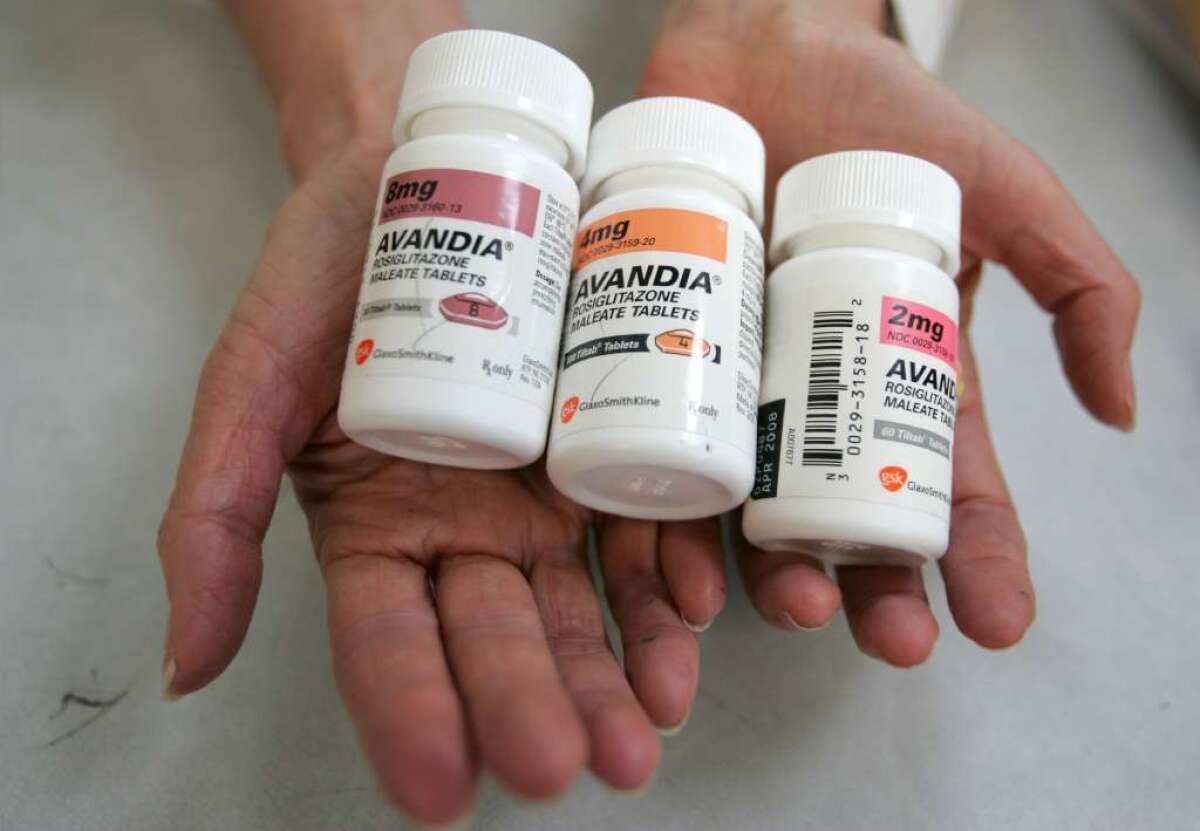
Avandia, a controversial diabetes drug that was thought to increase patients’ risk of heart attacks and other cardiovascular problems, has been cleared for widespread use by the Food and Drug Administration.
Federal regulators said Monday that the drug was no more dangerous than other diabetes medications and that it should be available to all patients without its current restrictions.
“Our actions today reflect the most current scientific knowledge about the risks and benefits of this drug,” Dr. Janet Woodcock, director of the FDA’s Center for Drug Evaluation and Research, said in a statement. “Given these new results, our level of concern is considerably reduced.”
GlaxoSmithKline, the company that makes Avandia, said it welcomed the FDA’s decision.
“GSK maintains its view that Avandia is a safe and effective treatment for Type 2 diabetes when used appropriately,” the London-based company said in a statement.
Avandia used to be the top-selling drug for patients with Type 2 diabetes, a disease that causes people to lose their ability to metabolize blood sugar with insulin. Unlike other drugs that help patients produce more insulin, Avandia works by making cells more sensitive to insulin, allowing the body to use it more efficiently.
The drug, whose generic name is rosiglitazone, came on the market in 1999 and had more than 1 million users in 2006. But in 2007, cardiologists warned that patients taking Avandia were more likely to have serious heart problems than patients taking other diabetes drugs.
A study led by Dr. Steven Nissen of the Cleveland Clinic linked Avandia to a 43% increased risk of having a heart attack and a 64% increased risk of death due to heart disease. The findings, based on data pooled from 42 small clinical trials, were published in the prestigious New England Journal of Medicine.
Those results prompted some experts to question how well the FDA was monitoring the safety of prescription drugs. Just a few years earlier, the federal agency withdrew its approval of the painkiller Vioxx after it evidence emerged that it doubled the risk of heart attacks and strokes. When the critique of Avandia emerged, three major congressional committees announced their intention to investigate.
The FDA responded by adding warning labels to Avandia.
After further study, the agency said in 2010 that patients taking Avandia were 30% to 40% more likely to develop a condition called myocardial ischemia, which decreases blood flow to the heart. Those who were also taking insulin or drugs to control their blood pressure faced a greater risk, the scientists said.
Regulators imposed a host of restrictions on the drug. New prescriptions could be written only for patients who could not control their blood sugar with other medications, including Avandia’s main rival Actos. Patients who were already taking Avandia were required to sign an informed consent statement acknowledging that they are aware of the drug’s heart risks. Regulators in Europe pulled it off the shelves altogether.
Today, only about 3,000 Americans still take Avandia, and they are closely monitored by the FDA and GlaxoSmithKline.
A new analysis this year by an FDA advisory panel suggested the initial concerns about Avandia were overblown. Members of the panel pointed to design flaws in Nissen’s study and said their evaluation found no evidence that the drug made patients more vulnerable to heart attacks or other heart problems.
The initial “signal of increased risk of heart attacks” reported by Nissen in 2007 has not been confirmed, the FDA said in the statement released Monday.
As a result, the FDA will no longer require doctors to limit Avandia prescriptions to certain patients. Its new label will likely state that anyone with Type 2 diabetes can use the drug in combination with diet and exercise to control their blood sugar, the agency said.
Patients who take Avandia will no longer be required to participate in a tracking program, and they’ll be able to get the drug through regular pharmacies.
The FDA also said it would no longer require GlaxoSmithKline to conduct a clinical trial comparing Avandia to Actos and to other diabetes drugs.
Monday’s vote of confidence from regulators won’t be enough to rehabilitate Avandia’s poor reputation among physicians, experts said.
“I don’t believe people are going to flock back to it,” said Dr. Karol Watson, a cardiologist and co-director of UCLA’s Program in Preventive Cardiology.
“People just weren’t prescribing it because there were a lot of other alternatives that could do the same thing and without the hassle,” she said, adding that new diabetes drugs have been developed since Avandia’s troubles began.
Gerald van Belle, a clinical trial expert who served on the FDA advisory panel, urged the agency in June to keep the restrictions on Avandia in place. On Monday, he reiterated his concerns about making the drug widely available.
“Since this is a safety issue, I think it pays to be cautious,” said Van Belle, a professor of biostatistics at the University of Washington in Seattle.
“There were two new observational studies that suggested risk, as before,” he said. Without clinical trials that unequivocally demonstrate Avandia’s safety, the drug’s use should continue to be highly restricted, he added.
Avandia is also sold in combination with the diabetes drugs metformin and glimepiride under the brand names Avandamet and Avandaryl. Restrictions on those drugs will be lifted as well, the FDA said.
Times staff writers Monte Morin and Melissa Healy contributed to this report.
If you’re interested in the latest medical research news, you like the things I write about. Follow me on Twitter and “like” Los Angeles Times Science & Health on Facebook.
ALSO:
FDA panel recommends continued Avandia sales, with restrictions
Bad news on red meat: Eating more is linked to diabetes risk
Gut microbes again linked to diabetes -- but geography matters