U.S. Health Officials Issue Anthrax Vaccine for Those Exposed to Bacteria
Top federal health officials released the anthrax vaccine Tuesday as an experimental treatment for people exposed to the deadly spores, out of concern that bacteria lingering in their lungs may still make them sick.
This is the first time the vaccine--mandatory for troops serving in the Persian Gulf War--will be made available for treatment. The vaccine previously had been used only to inoculate individuals before possible exposure, either on the battlefield or in laboratories.
The decision by Health and Human Services Secretary Tommy G. Thompson allowing the new use may have long-term implications for public health response, opening the door to vaccinating victims in the aftermath of bioterrorist attacks.
Nearly 10,000 people, all of whom were advised to take a 60-day cycle of antibiotics to prevent anthrax, are eligible to receive the vaccine. Health officials estimate only 3,000 are at high risk of contracting the disease.
The vaccine’s use is considered experimental because it has not been approved for anthrax treatment. It requires three doses over four weeks before taking effect, making it necessary for recipients to stay on antibiotics during that time.
An additional 40 days of antibiotics also have been made available to those who want continued protection but choose not to be vaccinated. Health officials said another option is for people to finish their original course of antibiotics and then be closely monitored for signs of illness.
Federal health officials stopped short of recommending the vaccine outright for mail workers and Capitol Hill staffers thought to be at the highest risk, citing too little evidence that the benefits of the vaccine outweigh the risks.
The lack of clear direction from HHS about use of the vaccine caused concern among some local health officials and postal leaders.
Dr. D.A. Henderson, HHS’s director of public health preparedness, said that, once again, health officials are faced with “difficult decisions based on really inadequate information.”
“If this were a vaccine which . . . had no associated reactions [and] would work very well, that would be one thing,” he said, “but this vaccine does have reactions associated with it, so there’s a negative side to it.”
Among known side effects are swelling and rashes--caused by the shot itself--that can be “quite dramatic,” said Kathryn Zoon, director of the Food and Drug Administration’s center for biologics.
Health officials and postal authorities also said they were concerned that not enough is known about the effects of the vaccine on a work force that is neither as young nor as healthy as military troops.
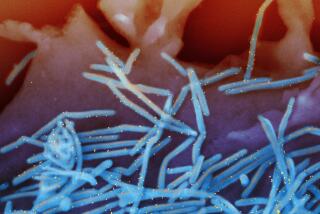
Anthrax, a rare disease in the U.S., has killed five people and sickened at least 13 others since a bioterrorist attack was launched by mail in September.
Public health officials had originally rejected using the vaccine to treat those possibly exposed to the bacteria, although what little anthrax research that had been done indicated it would protect against later onset of disease. The 60-day window for antibiotics was set in part because of a previous outbreak in Russia that included a case of anthrax developing in a person 43 days after initial exposure.
The latest escalation in the public health response comes as health officials have grown increasingly concerned about lingering risks. They have been troubled by the deaths of two women with no known exposure to anthrax and by new research that shows the opening of the letter sent to Sen. Tom Daschle (D-S.D.) may have released thousands of lethal doses of anthrax.
The disease is caused by tiny spores that can remain dormant in the lungs for long periods--potentially activating and infecting someone after antibiotic treatment ends.
Health officials faced heated criticism for their early response to the attacks. While they moved quickly to treat workers on Capitol Hill, where the anthrax-laced letter to Daschle was opened Oct. 15, the postal facilities that processed contaminated letters remained open and postal employees were told they were safe.
Two mail workers at the Brentwood postal hub in Washington, D.C., died of anthrax within the week; five additional mail workers in Washington and New Jersey fell ill with inhalation anthrax but recovered.
Acting out of what he called “an abundance of caution,” Thompson said Tuesday that the vaccine would be available for high-risk workers who consented to the vaccination and agreed to participate in a study. Those who choose that option could start vaccinations as soon as today.
About 10,000 doses of top-quality vaccine have been sold to HHS by the Defense Department in recent weeks. If all eligible people elected to take the vaccine, HHS would need about 20,000 additional doses. Health officials said Tuesday they do not anticipate the need to be that high but are prepared to get additional supplies if necessary.
An additional 209,000 doses of vaccine from an older batch that does not meet FDA standards can be used only in an emergency.
As a condition of sale, HHS officials indemnified the vaccine’s maker from litigation. The anthrax vaccine is already the subject of several pending lawsuits by Persian Gulf War veterans who believe it is responsible for their illnesses.
Some outside experts as well as military officials who have researched Gulf War syndrome, however, say the vaccine is no more dangerous than any other vaccine.
Postal officials expressed concern Tuesday about the logistics of vaccinating their employees and emphasized that the vaccine’s release did not come at their request.
“Capitol Hill has a very small number of individuals to deal with,” said Kristen Krathwohl, a Postal Service spokeswoman. “They have a military doctor on site who, as soon as there is a protocol in place, can begin following it. We don’t.”
More to Read
Start your day right
Sign up for Essential California for news, features and recommendations from the L.A. Times and beyond in your inbox six days a week.
You may occasionally receive promotional content from the Los Angeles Times.