FDA advises Zika screening for all U.S. blood centers
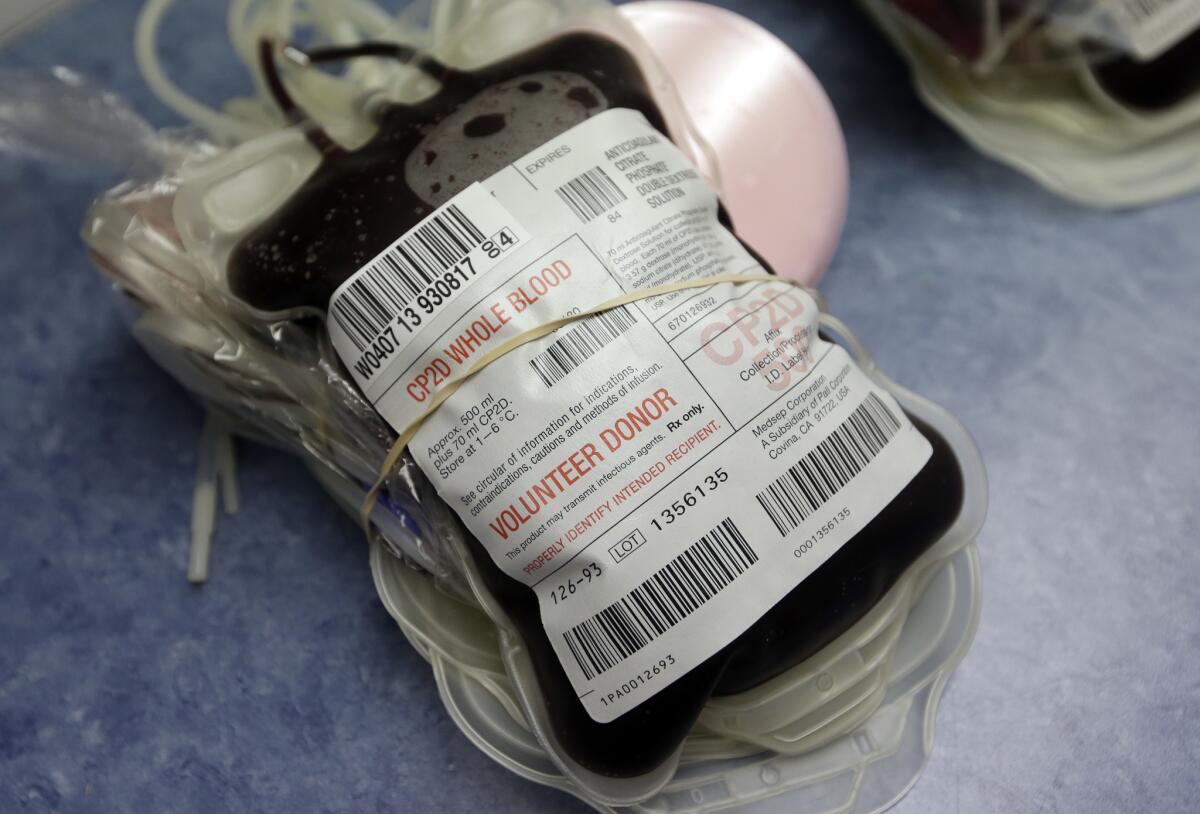
The Food and Drug Administration wants all U.S. blood centers to start screening for Zika, a major expansion intended to protect the nation’s blood supply from the mosquito-borne virus.
The new advisory means all U.S. states and territories will need to begin testing blood donations for Zika. Previously, the FDA had limited the requirement to Puerto Rico and two Florida counties.
“There is still much uncertainty regarding the nature and extent of Zika virus transmission,” said Dr. Peter Marks, director of the FDA’s biologic products center, in an agency release. “At this time, the recommendation for testing the entire blood supply will help ensure that safe blood is available for all individuals who might need transfusion.”
Blood collection sites already test donations for HIV, hepatitis, West Nile virus and other blood-borne viruses.
Zika is a mosquito-borne illness that has spread through dozens of countries in Latin America, the Caribbean, and now the United States.
FDA officials said Zika testing is already underway in Puerto Rico and parts of Florida, where “it has shown to be beneficial in identifying donations infected with Zika virus.”
The FDA has authorized use of two experimental blood-screening tests for Zika, one made by Roche and another from Hologic Inc. Several testing sites are already voluntarily using the technology, including blood centers in Texas. The cost of adding Zika testing to the blood screening process is less than $10, according to officials at South Texas Blood and Tissue Center.
Since February, U.S. blood centers have been turning away people who have traveled recently to areas with Zika outbreaks, under a previous FDA directive.
Zika is spread primarily by mosquito bites, as well as sex. There have been cases of Zika transmission through blood transfusion in Brazil.
The FDA works with other federal agencies to set standards for screening, testing and handling blood donations.
Last month, blood centers in Miami and Fort Lauderdale had to halt donations until they could begin screening each unit of blood. The order followed now-confirmed reports of local Zika transmission in the Miami area — the first in the continental U.S.
Puerto Rico suspended blood donations and imported blood products in March until the island began screening its blood.
Friday’s announcement follows recent pressure from members of Congress urging the FDA to expand Zika screening.
“We must implement widespread universal screening now to prevent any further contamination of the blood supply before it occurs and to pre-empt a widespread shortfall in the blood supply,” said Reps. Lloyd Doggett, D-Texas, Patrick Murphy, D-Fla., and half a dozen other House members, in a letter to the FDA earlier this month.
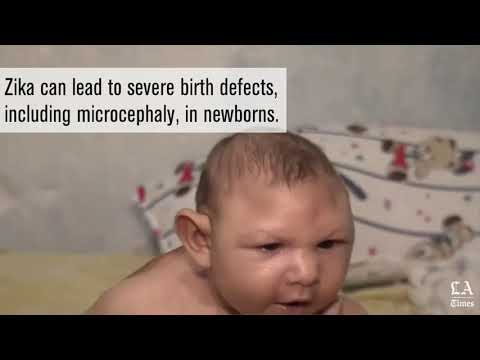
The Zika virus causes only a mild illness in most people, but scientists have confirmed that infection during pregnancy can lead to severe brain-related birth defects.
The tropical mosquito that spreads Zika and other viruses is found in the southern U.S. While health officials have predicted that mosquitoes in the continental U.S. would begin spreading Zika this summer, they also have said they expect only isolated clusters of infections and not widespread outbreaks. There have been about 40 cases of homegrown Zika in Florida.
MORE ON ZIKA
Before Zika: How the U.S. fought illnesses from pests in the past
From ‘shrines’ to head-to-toe attire: How Miami is coping with the arrival of Zika
Three vaccines prevent Zika infection in monkeys; vaccine trial in humans gets underway
More to Read
Start your day right
Sign up for Essential California for news, features and recommendations from the L.A. Times and beyond in your inbox six days a week.
You may occasionally receive promotional content from the Los Angeles Times.