Brain’s Darwin Machine
LA JOLLA, Calif. — Alysson Muotri was looking for brain cells that glow in the dark.
With growing frustration, the 31-year-old Brazilian cancer biologist stared through his microscope at slides of brain tissue for any evidence his experiment had succeeded. His eyes ached.
Maria Marchetto, 28, took pity on her husband. Let me look, she said. In a darkened room at the Salk Institute for Biological Studies here, she began to scrutinize the tissue samples for firefly flecks of fluorescent light.
Together, the couple stalked an elusive sequence of DNA hidden in the heredity of every human cell. The wayward strand appeared to seek out developing brain cells and, like a virus, arbitrarily alter their genetic makeup.
In this way, it might be partly responsible for the infinite variety of the mind.
In debates over creationist doctrines, evolutionary biologists often are hard-pressed to explain how nature could make something as intricate as the human brain. Even Alfred Wallace, the 19th century biologist who discovered natural selection with Charles Darwin, could not accept that such a flexible organ of learning and thought could emerge by trial and error.
No two brains are exactly alike, despite their overall anatomical similarity. Each brain changes throughout a lifetime, altered by experience and aging. Even the simplest mental activities, such as watching a moving dot, can involve slightly different areas in different people’s brains, studies show.
Underlying every personal difference in thought, attitude and ability is an astonishing variety of brain cells, scientists have discovered.
Some neurons fire only when they perceive a straight vertical line, others when they are exposed to a right angle. Some respond to the emotions in a facial expression or to social cues. Others retain a memory long after conscious recollection has faded.
To respond so selectively to experience, each of these cells must vary incrementally from its neighbors, as singular as a face in a crowd.
Yet what could generate such diversity?
If Muotri’s suspicion was correct, a peculiar string of biochemicals caused the billions of neurons in each person’s brain to develop in distinctly different ways, so that even identical twins could develop minds of their own.
Muotri and Marchetto searched hundreds of slides for any sign that the DNA sequence had altered brain cells. Each tissue sample took an hour to analyze under ultraviolet light.
When Marchetto closed her eyes, she could see the glowing afterimage of neurons.
The spidery cells, she would say later, crawled through her sleep.
In every human brain, there are as many neurons as there are galaxies in the known universe — about 100 billion, drawn from 10,000 different cell types and woven into a three-dimensional tapestry, with threads of neural interconnections that number in the trillions.
Each one is tinder for the spark-of-life experience.
Memories are made of this gray matter. So are inspiration and imagination.
Electrochemical currents of intellect and emotion race though living labyrinths of neurons at 200 mph. When they are blocked, diverted or damaged, abilities atrophy. Personality disintegrates.
By exploring the life and death of these cells, researchers hope to learn how biochemistry becomes thought.
Among the molecules of mental life, they are finding signs of an evolutionary struggle for survival.
In the womb, brain cells increase at a rate of 250,000 a minute. The total doubles after birth. By age 3, a child’s brain, on average, has twice as many neurons and neural connections, and is twice as energetic, as an adult’s.
Already, a ruthless competition is underway.
Throughout developing brain circuits, neurons and synapses vie for sensory stimulation — the electricity of touch, vision, taste, hearing and smell. Some thrive, while others atrophy for want of exposure to life’s raw experience, to be eliminated at a rate of thousands per second.
By adulthood, more than half the neurons a brain possessed in early childhood will have died.
For many years, scientists were convinced that the brain quickly lost its ability to produce new neurons. But in the last decade, independent research teams at the Salk Institute led by Fred W. Gage and at Princeton University by Elizabeth Gould showed that even middle-aged minds generated thousands of new neurons every day in areas crucial to learning and memory.
Inside the Darwin machine of the brain, therefore, the survival struggle of neurons and synapses lasts a lifetime.
In this competition, the forces of variation and selection that shape a species also sculpt each brain, neuron by neuron, creating the biological truth of individuality.
“The neurons are never identical,” Muotri would say. “They are all slightly different.”
Not so long ago, scientists were certain that genes dictated everything about the brain. But when researchers successfully analyzed the complete human genome three years ago, they discovered that it contained only 25,000 genes — not the 100,000 they had predicted.
Indeed, less than 3% of the genome contained functional genes.
There wasn’t nearly enough information in them to account for so many different brain cells and synapses.
Something else had to be at work. But what?
At the Salk Institute, Gage, 53, was consumed by the mystery of the new neurons he had discovered.
In the brain’s unexpected ability to renew itself, he saw the potential for repairing brain damage from maladies such as Alzheimer’s disease or spinal cord paralysis.
Gage — boyish, unfailingly affable, with a trim, sand-colored mustache and a wave of blond hair that crested over a high forehead — was among the most influential neuroscientists of his generation. He orchestrated his laboratory’s research efforts the way an impresario manages an opera company, artfully matching the ambitions of 30 scientists to questions that best challenged their abilities.
Gage deployed tools all but unheard of a generation ago — computerized gene micro-arrays, automated gene sequencers, genetically engineered animals.
His working arrangements were also at the cutting edge.
One section of his Salk laboratory was set aside for experiments with stem cells from human embryos, where work could proceed independent of federal funding and unencumbered by federal policies that restrict such research.
In 2003, he co-founded Brain Cells Inc. to exploit his discovery that humans generate new brain cells throughout life.
Almost immediately, two staff scientists in his Salk laboratory found that a curious DNA sequence muddled their efforts to discover how neural stem cells produced new neurons.
In one such experiment, the researcher hoped to learn how a particular gene affected the life cycle of a neuron. She altered mouse embryos to deactivate the gene.
At first, these artificial rodents seemed normal enough. Yet upon close study, some of the creatures seemed dimwitted. A few had memory problems. Others had trouble learning.
Intrigued, the scientist compared the genetically engineered neurons to natural cells. The only major difference she could detect was the activity of this puzzling genetic sequence.
Her experiments had taken two years. Crestfallen, she turned her attention elsewhere.
“At the time, we could not make heads or tails of it,” Gage recalled. “We would have long discussions, but I could not get anyone interested in working on this.”
Scientists called the curious genetic sequence a “jumping gene.”
It could move up and down the double helix of DNA to insert itself into the genetic structure, like a black snake crawling along a branch into a bird’s nest.
Despite the name, the sequence was not a gene but a primitive precursor — called a long interspersed nuclear element, or LINE — that struggled for survival inside the microcosm of a cell. The LINE sequence belongs to a mysterious family of mobile genetic elements called retrotransposons.
For 600 million years, it existed solely to copy itself.
All mammals contain such LINE sequences. But as species became more intelligent, they retained fewer types. No one knew why.
Mice harbored 3,000 different kinds of LINE elements, rats 500. Humans had about 100 types that differed from one person to the next.
All told, there are as many as 850,000 copies of such junk DNA in the human genetic structure, composing almost half of every cell’s heredity. Most researchers dismissed it all as the detritus of parasites, viral infections and evolution’s failed experiments.
Unlike the other molecular relics littering the human genetic code, however, the particular human sequence that cropped up in the Salk laboratory, called an L1 LINE, was still on the move.
So many thousands of times had it copied itself into the human genome that it now made up one-fifth of a cell’s DNA.
Most copies were stranded far from any functional gene. Many were truncated, broken off like an aria interrupted by a cough.
Every once in a while, the sequence landed close enough to a gene to disrupt its behavior or change its expression.
A single jumping retrotransposon is the reason that Great Danes, dachshunds, border collies and certain other domestic dogs have patchy black-and-tan coats, researchers at Texas A&M University recently reported. It landed in a gene that affects the color of dog hair.
But no one had ever heard of these DNA strands reweaving the genetic fabric of individual brain cells.
Gage asked Muotri to look into it. The Brazilian was a cancer geneticist, not a neurobiologist like most of the researchers in the Gage lab.
Gage had recruited him to study brain diseases, drawn by his intellectual energy and persistent curiosity. When that project failed to materialize as expected, Muotri was open to a new question, even one outside his immediate field.
The idea of jumping genes didn’t seem so strange to him. Such mutations were a rare cause of genetic disorders such as hemophilia and Duchenne muscular dystrophy.
In a gray T-shirt, shorts and flip-flops, Muotri had the look of someone who came to La Jolla for the surf, not the science. He wore a carving of a hammerhead shark on a chain around his neck and a watch with three dials on his right wrist.
“I felt like an odd fish in the aquarium,” Muotri would say later. “I decided to look with the cancer mind-set. Maybe I [would] learn something.”
Gage invested more than $1 million in private funds, time and laboratory resources in the experiment.
No federal agency would fund it.
“They probably think I’d gone off the deep end,” Gage said later. “It was too wild.”
Muotri and Gage wanted to know whether the L1 sequence was actually moving around in developing brain cells.
Normally, the sequence copied itself into reproductive cells in the testes and ovaries, where a randomly remodeled gene might be passed to succeeding generations. The sequence did not seem active in any other type of cell in the body.
They could not experiment on people, so they inserted the human DNA into a custom-made brood of mice.
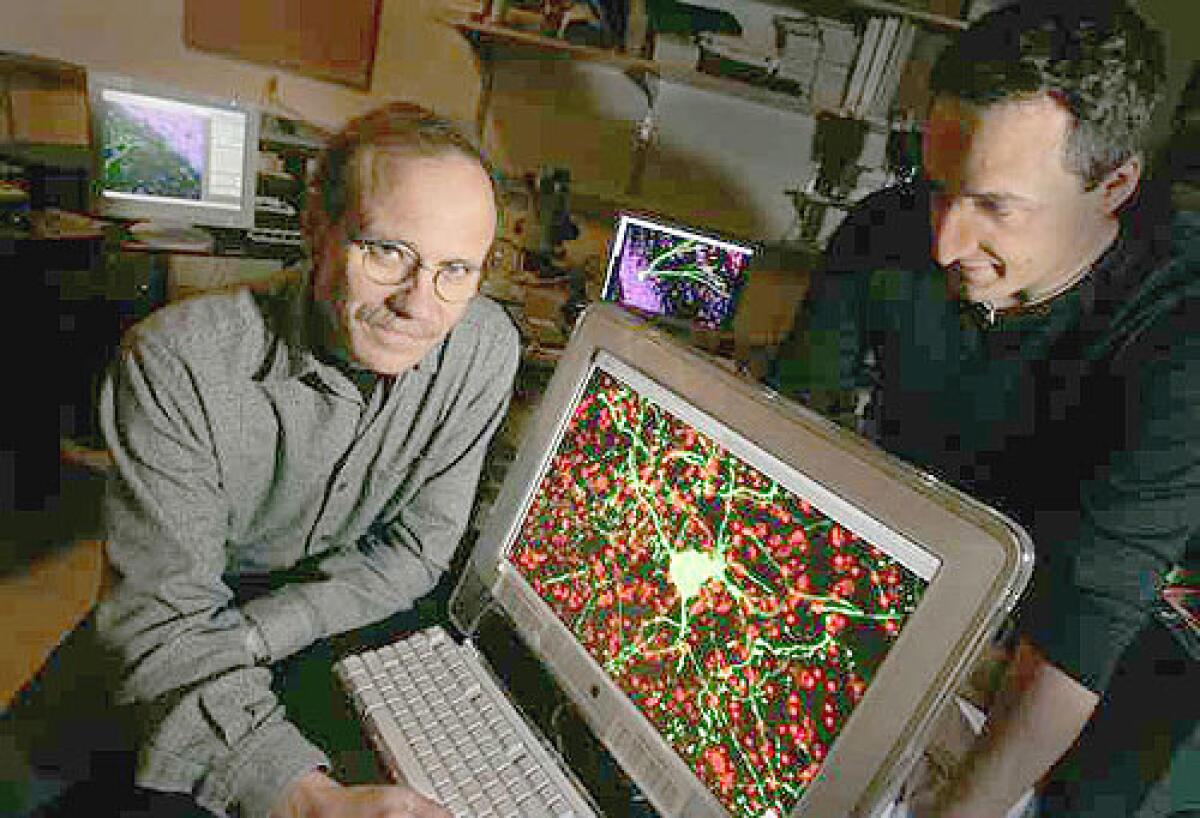
To make the L1 sequence visible under a microscope, Muotri and his colleagues added to it a molecular tracer — a green fluorescent protein — that would light up whenever the DNA intruder entered a growing cell.
With a splinter of hollow glass, Muotri injected the sequence into mouse eggs, then transferred them into female mice, where he hoped the new DNA would take hold in growing embryos.
Of seven brown mice in the litter, two contained the altered human DNA.
He bred those with wild mice to create a family in which the L1 sequence was poised to jump into any cell of the body. He ended up with 20 transgenic mice.
To search for evidence of brain activity, he sliced each mouse into wafers 40 microns thick and mounted tissue from every organ on slides.
If the sequence had jumped anywhere, it should reveal itself, like a firefly at midnight, with a fluorescent glow.
Searching the slides under ultraviolet black light was such an eyestrain that Muotri could only keep it up for about four hours a day. At the same time, the fluorescence was depleted by exposure to ultraviolet. So the longer he looked, the fainter the light became.
During a break halfway through one scanning session, Muotri browsed research articles in the Proceedings of the National Academy of Sciences. He stiffened.
At the University of Pennsylvania, a rival research team had already conducted his experiment and published the results: They examined the entire animal for signs that the sequence was jumping from cell to cell outside reproductive organs but failed to find any evidence of the brain activity that Muotri sought.
“They checked skin, they checked liver, they checked everything including the brain,” Muotri recalled. “They looked for the same thing I was looking for and could not find it. They reported exactly the result I did not want to see.”
The Brazilian brooded.
By 2005, he had spent two years chasing the L1 sequence. His fellowship would run out soon. Should he abandon the experiment? What was the likelihood he might find something that skilled competitors had overlooked?
He ought to finish what he started, he decided, no matter how futile the effort.
His wife lent a hand.
Marchetto — red tank top, blue jeans and yellow hair — was a splash of primary colors against the laboratory’s gray concrete walls. She knew little about neural anatomy. Her doctorate was in skin cancer biology. But she was more meticulous than her husband.
As her eyes learned the microscopic maze of synapses and support cells, she could see a glow inside the translucent spheres of brain cells.
“It was, like, crazy green,” she recalled. First one cell, then five, 10, a dozen.
She found the fireflies in the brain.
In the black light of the microscopy room, her brilliant smile was like the moon emerging from the clouds.
“Please,” she said, calling her husband to the microscope. “This is a neuron.”
They caught it in the act.
To their wonder, the L1 sequence had left its distinctive mark wherever they looked in the mouse brains — throughout areas devoted to memory, learning, emotion, motor control and the senses.
They discovered that the sequence affected only developing brain cells. It also seemed to home in on neural genes, arbitrarily changing their behavior. Every time it affected a gene, it set that neuron apart from its neighbors in the brain and from all other cells in the body.
In the mouse experiment, the sequence jumped into one of every 100 brain cells.
Unpublished data from follow-up experiments by colleagues suggest that in human cells, the sequence jumps into 80 of every 100 neurons.
“Every neuron may have a different genetic profile,” Muotri said. “Almost all the cells have at least one L1 insertion.”
The researchers were elated but puzzled.
From the standpoint of conventional evolutionary theory, any independent genetic change in a neuron was a dead end. The random changes caused by L1 inside a brain cell could never be passed on directly through the genetic shuffle of sex.
At this point, Muotri and Gage had an audacious thought.
Perhaps the sequence, striving for its own survival inside the growing neuron, made the brain more responsive to changing circumstances. Had natural selection seized on the one rogue sequence most useful for crafting an infinitely adaptable human brain?
“There are subtle differences in everything we do throughout our lives,” Gage said. “Maybe this is how we generate a deeper adaptability to deal with the unexpected.
“We believe the sequence is generating this diversity to fine-tune the brain.”
*
MAPPING THE MIND
One in a series of occasional articles about scientists’ efforts to explore the creation of beliefs and behavior in the synapses of the brain. To read previous articles in this series, go to latimes.com/mind. For an archive of Column One articles, visit latimes.com/columnone.



