Chaos and competition for vital coronavirus test could slow reopening of the economy

As debate intensifies about how and when the country can resume regular life, immunity testing widely seen as essential for that reopening is mired in the same competition and chaos that marred earlier diagnostic tests.
“It’s already a case of the continuing wild, wild West,” said David Relman, a professor of microbiology and immunology at Stanford who is on a White House advisory panel on testing. “The sequel is certainly live and in action.”
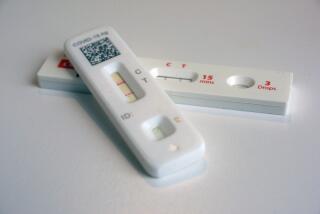
Dozens of companies have rushed tests onto the market that promise to tell users whether they have been exposed to the virus, and therefore may have some kind of immunity.
These serology tests, which look for antibodies in blood that have the ability to fight off the virus, are rolling out at a rapid pace both for private individuals looking for hope and clarity, and public health officials increasingly pressured for timelines for reducing social restrictions.
They range from simple sticks that work like a pregnancy test and rely on a few drops of blood, to complex lab tests that use the live virus and can be performed only in high-security settings.
But the tests may face much of the same lack of availability as their diagnostic counterparts. In some cases, the screenings require the same mixing liquids that are in short supply for those earlier tests, potentially sparking another round of fierce competition between states, cities and counties — and even the federal government — all vying for the same limited resources as more places begin immunity testing.
Hundreds of thousands of L.A. County residents may have been infected with the coronavirus by early April, outpacing total of known cases, report says.
“We are going to have shortages,” said Thomas Denny, professor of medicine and global health at Duke University who has started a pilot program in North Carolina using a European-made test.
Denny recently spent $45,000 on kits to test up to 4,000 people in an attempt to determine how long the virus may have been circulating in his state. But he is uncertain how quickly he will be able to get more tests for subsequent rounds of bloodwork because of the demand.
“I think we may be able keep the work going but not with the speed we’d like,” Denny said. “This is same problem we went through scaling up for the diagnostic testing.”
The reliability of the tests is also unknown, creating a marketplace where buyers should beware, with questions of balancing need with uncertainty.
To avoid the seething criticism it faced on red tape and mishaps involving diagnostic testing, the Food and Drug Administration removed many rules for the approval of serology tests. The result has been more than 90 tests from manufacturers worldwide hitting U.S. shores with little data to back up their claims.
The problems with the tests range from high percentages of false results to a lack of scientific certainty about what antibodies mean when they are present in accurate tests.
The latest maps and charts on the spread of COVID-19 in California.
While most scientists are hopeful the presence of antibodies does bring some immunity, no one yet knows how long it could last or if it’s possible to get the virus more than once. That makes it difficult to know what the tests mean, and how to manage public expectations.
“Just because you have antibodies doesn’t mean you are immune,” said Michael Gunn, professor of medicine at Duke University. “It’s just too early for us to have figured out what the reliable markers are for true immunity.”
Relman and others said that along with questions about the tests themselves, the federal government has failed to use its muscle to coordinate community testing and supplies, increasing the potential for cutthroat competition.
As with finding ventilators and protective gear for medical workers, President Trump has been clear that it is up to the states to figure out procurement and how they use the tests and equipment they procure.
“There are things that you need to depend upon a federal system for providing and that would include standards and guidance,” Relman added. “Those really need to come from the top because you want everyone playing by the same set of rules.”
In the absence of federal help, there is little choice but for states and institutions to forge their own paths as pressure mounts for a return to normalcy, he said.
Newsom said that while areas across the state have been affected differently by the pandemic, the “virus knows no jurisdiction, knows no boundaries” and could easily spread into neighboring counties if they ease restrictions prematurely.
“It’s like waiting for Godot. You could sit around waiting for the CDC [Centers for Disease Control and Prevention] to figure this out and we would know zip,” Relman said.
Many states aren’t waiting. On Monday, New York announced its own program to randomly test 3,000 residents in an effort to better understand how far the virus has spread.
Dr. Mark Ghaly, California’s secretary of health and human services, said last week the state would move forward with more community testing in coming weeks, though details on where or how have not been released.
“Thank God California is rich” in scientific and technical experts, Ghaly said of the plans for more serology testing. “If we are ever going to understand what it means to relax and loosen, modify some of the various orders including the stay at home … you have to begin to understand prevalence and incidence of disease,” Ghaly said.
But Dr. Bob Kocher, an adjunct professor at Stanford University School of Medicine and a member of Gov. Gavin Newsom’s task force on testing, said the state is being selective as it sorts through the options, and hasn’t yet come up with recommendations on the majority of them.
“We want to validate them first,” he said.
Public health officials in places including Santa Clara and Los Angeles are already starting broader pilots of serology testing. Results released Monday of a first round of testing in Los Angeles found that the virus may have had greater community spread than previously known, a finding mirrored in Santa Clara.
Other medical systems including UC San Francisco and Stanford are also running surveys of patients and workers to provide better treatment and help identify which employees may have some immunity.
But each of those surveys are running independently, left to their own devices to choose and purchase tests. They are making those decisions largely based on their quick research on what works and what doesn’t, understanding that what they purchase today may not be available for future rounds of testing because of the competition.
Alan Wu, a professor of laboratory medicine and head of the clinical lab at Zuckerberg San Francisco General Hospital, said his facility is starting a serology testing program for patients and staff this week that uses an antibody test produced in China.
Though he has vetted the test over the last month, he still has questions because much remains unknown about the virus, including what antibodies mean.
“The test results I am producing, I don’t know if this produces immunity or not,” said Wu. But he said he believes that it ultimately provides useful information, even if it is not the “gold standard.”
Wu is also concerned whether he can continue getting a supply of kits for future testing. To be effective, the serology efforts need to retest people over months or longer to determine if they remain immune.
“The manufacturer is having difficulty getting [them] into the United States,” Wu said. “There are only a handful of planes that come in from Shanghai every day.”
Los Angeles is facing the same pressure.
Neeraj Sood, professor at USC’s Price School of Public Policy and lead researcher on the L.A. County serology test, said Monday that the county has “enough tests to do one more wave.” After that, the county is “actively looking to figure out how to get the next batch of tests.”
He also is unsure about the reliability of the tests; currently, the study is using a rapid antibody test from a supplier in Minnesota.
“We are looking for data from other scientists as well as from federal agencies like the CDC and the FDA, who are actively trying to test these dozens of test kits that are available in the market, to sift out the good ones from the bad ones,” Sood said. “So we are waiting for the data to come to figure out which are the best kits to use.”
More to Read
Start your day right
Sign up for Essential California for news, features and recommendations from the L.A. Times and beyond in your inbox six days a week.
You may occasionally receive promotional content from the Los Angeles Times.