Niche drugs become victims of their own success as off-label use leads to shortages
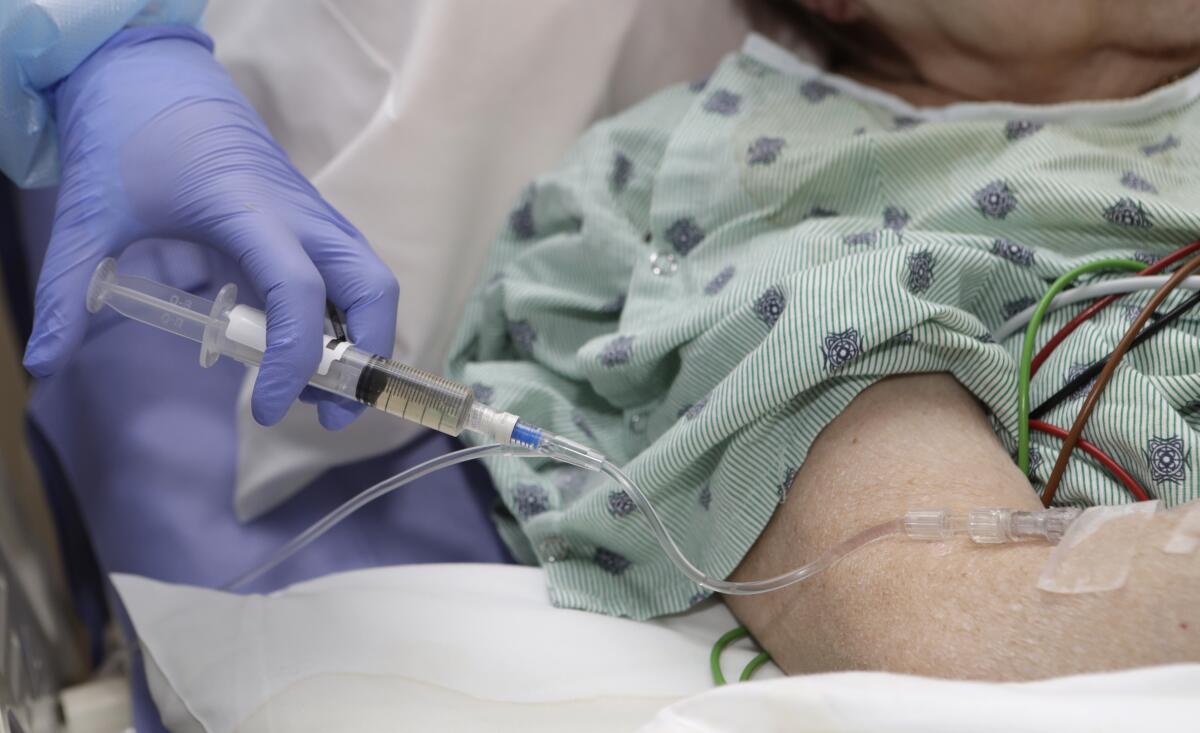
Medical treatment has knocked down tumors in 6-year-old Easton Daniels’ brain, but the drug wiped out his immune system as well.
To bolster his immune function and help keep him healthy, he has visited Cincinnati Children’s Hospital for intravenous infusions of immune globulin about once a month for the last year and a half.
But in early July, his family was stunned by a letter from the hospital: “All of Easton’s appointments canceled until further notice,” said his dad, Jeremy Daniels, who works in custodial services for a school.
Hospitals and clinics nationwide are reporting a shortage of the medication. Intravenous immune globulin, or IVIG, is used for a wide variety of medical conditions beyond those for which it was first targeted because it is rich in antibodies, which are proteins that help fight off infection. Some of these additional uses of IVIG have proved effective, and some have not.
With IVIG in short supply, hospitals are left to make tough choices about who receives it. The result is a type of triage like that faced by Easton’s family. Patients are finding themselves caught in a gray area over which conditions qualify.
“IVIG can be a useful treatment for evidence-based purposes, but it’s also often used as a last-chance, nothing-is-working Hail Mary kind of approach for myriad conditions even when there is not clear evidence that it helps the patient,” said Dr. Jerry Avorn, a physician who studies drug prescribing practices at Harvard Medical School.
Nationwide, drug shortages of all kinds — including antibiotics, heart drugs and saline solution — are becoming more common, according to the Food and Drug Administration. The shortages often result from manufacturing problems, such as when a factory shuts down or too few suppliers exist to meet demand.
But the reasons for shortages of expensive infused drugs are particularly complicated. Complex manufacturing processes, scientific uncertainty and financial motivations are all factors.
In the case of IVIG, the expensive treatment may be a victim of its own widening use.
Dating to the 1950s, immune globulin is often the only therapy for certain genetic, life-threatening conditions that disable the body’s infection-fighting function. Its intravenous form has been licensed by the FDA to treat six conditions, including primary immunodeficiencies; Kawasaki disease, which causes inflammation in the blood vessels; preventive care after bone marrow transplants; and a neurological condition called chronic inflammatory demyelinating polyneuropathy.
Today, IVIG is prescribed for patients whose immune systems have been compromised by viruses or cancer treatments, although there may be other medicines for reinvigorating the immune system in those cases. Prescribing a medicine for a purpose not approved by the FDA — known as off-label use — is legal and common. It sometimes leads to new and effective uses of a drug.
Some evidence shows that expanding the use of immune globulin for a wider variety of illnesses, including types of cancers or recurrent infections, is helpful. But it is also being tried for conditions “where it is ineffectual and may actually increase the risks to patients,” the American Academy of Allergy, Asthma and Immunology warned in March 2017.
In part as a result of this expanding off-label use, the Plasma Protein Therapeutics Assn. reports a 66% increase in distribution of the treatment from 2012 to 2018 across North America and Europe. Pharmacists and researchers who study drug shortages agree that increasing demand is playing a role.
Immune globulin takes up to a year to produce. It starts with the collection of blood plasma from healthy donors, and processing, packaging and shipping often occur at overseas manufacturing centers. There are several manufacturers, with combined global sales of about $22.6 billion, according to Zion Market Research.
Efforts to boost plasma collection could help deal with the shortfall. Hospitals are also key players, since they often decide how the drug is doled out to patients, said John Boyle, chief executive of the Immune Deficiency Foundation, a group that advocates on behalf of people with genetic defects of the immune system. “Hospitals use an enormous portion of the plasma products out there.”
Many are scrambling to come up with ways to stretch their supplies.
Some, like Cincinnati Children’s, give top priority to patients who have no alternatives — often those with primary immune deficiencies — and those who would find themselves in a life-threatening situation if they did not get the treatment.
Others, whose indications “were not as clear-cut or it was not necessarily dangerous to them to forgo it, were placed on the bottom of the list,” said Dr. Derek Wheeler, chief of staff at Cincinnati Children’s.
Shortages are not affecting every hospital or clinic. Facilities have contracts with specific distributors or manufacturers, each of which can have a different supply line.
Dr. Cristina Porch-Curren, an immunologist in Camarillo, said her patients have not run into problems getting the treatment, although one had to change brands. She is concerned about the increasing use of immune globulin for off-label conditions.
“Off-label doesn’t always mean bad. If you have someone who is really sick, with some terrible infection, on occasion that may be OK,” she said. But with limited supplies, she worries about growing interest by researchers and some physicians in using immune globulin for more widespread or ongoing conditions, such as dementia.
“That’s concerning, especially for patients with primary immune deficiencies” who have no other alternatives, she said.
Back in Cincinnati, a temporary solution has been found for Easton.
FFF Enterprises offered to supply his family with a different type of immune globulin after Easton’s father reached out to the company, which is one of the largest distributors of the therapy. Instead of an intravenous dose, it has given Easton a subcutaneous form, injected as a shot at home. His doctor approved the switch, Easton’s father said, and the drug distributor said it would pick up the cost if his insurer balks.
Appleby writes for Kaiser Health News, a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.






