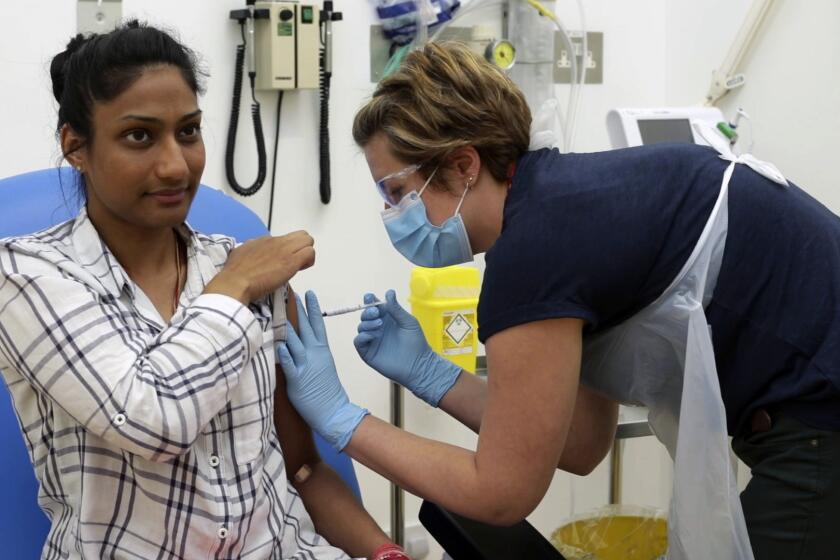
Clinical trial results indicate Moderna coronavirus vaccine is on the right track
Within 12 hours of getting the second dose of an experimental COVID-19 vaccine being developed by Moderna Inc., Ian Haydon began to feel chills. Then came nausea, headaches, muscle pain and delirium. He took his temperature: 103.2 degrees.
The 29-year-old Seattle resident, one of 45 participants in the company’s Phase 1 clinical trial, knew his body was trying to mount a defense against the vaccine. If it worked, his immune system would be primed to fight an actual coronavirus infection.
An aspiring marathon runner without any health issues, Haydon could not remember ever feeling this sick. His girlfriend took him to urgent care. Later that night, he fainted in their apartment.
With fluids and rest, the symptoms faded. A day and half after getting the shot, he felt fine.
Similar side effects of Moderna’s COVID-19 vaccine were described in a report published Tuesday by the New England Journal of Medicine. Its findings confirm the company’s preliminary announcement in May that the candidate vaccine prompted the production of coronavirus antibodies in human testers.
The results of the peer-reviewed study “are promising, and they support continued development of this vaccine,” Dr. Penny Heaton, chief executive of the Bill and Melinda Gates Medical Research Institute, wrote in an editorial for the journal. “However, we must bear in mind the complexity of vaccine development and the work still to be done before COVID-19 vaccines are widely available.”
Moderna is a front-runner in the global race to develop a vaccine and bring the COVID-19 pandemic under control. The 10-year-old Cambridge, Mass., company is one of six contenders to receive funding as part of Operation Warp Speed, a multibillion-dollar federal initiative designed to expedite vaccine development.
Its vaccine introduces genetic material from the coronavirus into the body, which responds by producing a protein that allows the virus to infiltrate a host cell. The presence of that so-called spike protein triggers the body’s immune response and induces the development of protective antibodies that can stop the virus in its tracks.
The technique, known as mRNA, was first developed to fight other coronaviruses — MERS and SARS — but had not been advanced beyond early clinical trials.
Can a coronavirus vaccine really be developed this year? Dr. Anthony Fauci and other experts say it’s possible but far from certain.
In the new Phase 1 trial, Haydon and the other study subjects produced coronavirus antibodies at levels comparable to patients who had contracted and recovered from COVID-19. However, that does not necessarily mean that the vaccine can provide immunity to the disease.
“It’s first step, but it is an exciting first step,” said Dr. William Schaffner, an infectious disease expert at Vanderbilt University and the medical director of the National Foundation for Infectious Diseases. “It showed that this novel technology — mRNA — can induce a substantial immune response. We trust that the immune response will correlate with protection down the road.”
Dr. Paul Offit, a vaccine expert at Children’s Hospital of Philadelphia, was more measured in his assessment.
“It was a small dose-range trial that showed that doses of vaccine were safe, and they induced a neutralizing antibody response similar to natural infection,” he said. “But we need a large Phase 3 trial.”
Moderna recently finished enrolling candidates for Phase 2 study, and its Phase 3 trials are expected to start later this month.
During the Phase 1 safety trial, the vaccine was administered in three doses — 25 micrograms, 100 mcg and 250 mcg — to three groups of 15 individuals. The men and women lived in Seattle and Atlanta and were between the ages of 28 and 41.
Although the study authors, led by Dr. Lisa Jackson of the Kaiser Permanente Washington Health Research Institute in Seattle, reported “no serious adverse events” or hospitalizations, each group of candidates experienced side effects that were significantly worse after the second dose.
Such side effects are not unusual, Schaffner said, citing the shingles vaccine.
If the Moderna vaccine winds up in widespread use, he said, “we’ll have to let everyone know in advance that your arm may hurt, and you will feel out of sorts for a day or so. We have to be aware of this too because these conditions can mimic aspect influenza or COVID itself.”
Three people who received 250 micrograms had their symptoms graded as “severe.” Moderna is no longer administering the 250-microgram dose in its trials.
The global race for a coronavirus vaccine involves a few basic approaches. Some have been around for decades, others are being tried for the first time.
Moderna is one of many biotech companies that have made a coronavirus vaccine a top priority, and investors have rewarded it. The company’s stock has risen from $18.59 on Feb. 24, when it announced it was pursuing a vaccine, to $75.04 at the close of markets on Tuesday. It jumped an additional $12.06 to $87.10 in after-hours trading following the study’s release.
With the ambitious goal of producing 300 million doses of vaccine by January, Operation Warp Speed will be challenged by logistics. Even if a vaccine is proven to be effective against COVID-19, production and distribution will be daunting.
“Remember, on Day One, we won’t have enough vaccine for all of the U.S.,” Schaffner said. “We will be rolling out a vaccine program as the vaccine becomes more available, and for a considerable period of time much of U.S. population will not be vaccinated. We will have to continue social distancing activities.”
As Moderna pushes ahead with new phases of its clinical trials, Haydon, who received the 250-mcg dose, has slowly resumed his normal routine. He has started marathon training again with the hope of running his first race by the end of the year, and he periodically has his blood drawn to measure the level of antibodies.
But he is not taking any chances. He still wears a mask, mostly stays inside and washes his hands “like a maniac.”
“I don’t feel protected,” he said. “My concern is actually to not get exposed, even more so than before. What if I were to catch full-blown COVID? I imagine hope for this vaccine would plummet.”