Gene-editing breakthrough found to minimize hearing loss in mice could help humans
Scientists are one step closer to using CRISPR-Cas9 to cure some types of hearing loss.
In a paper published Wednesday in Nature, researchers describe how they used the CRISPR-Cas9 complex to alter a faulty gene associated with a form of inherited, progressive hearing loss in the tiny ears of newborn mice.
Eight weeks after receiving the treatment, the mice were able to hear significantly better than their untreated peers.
Specifically, the treated mice could hear at the level of a quiet conversation, while the untreated mice could hear at the level of an old fashioned garbage disposal.
The authors said that while the new work is promising, much more research needs to be done before they know if the same technique will work in humans.
“Sometimes the mouse model is a good approximation of how the human patient behaves and sometimes not,” said David Liu, a professor in the department of chemistry and chemical biology at Harvard University who led the work.
Still, he said there are reasons to be optimistic. In part, that’s because the same mutation leads to progressive hearing loss in both mice and humans.
Previous studies have shown that genetic factors are responsible for nearly half of all cases of deafness.
In addition, approximately 20% of genes associated with genetic deafness are dominantly inherited. That means that if you get one good copy of the gene from one parent and one bad copy from the other, it’s the bad gene that will prevail.
In this study, the authors worked with a strain of mice known as the Beethoven strain, after the famous composer who suffered progressive hearing loss over the course of his life.
Beethoven mice have a single erroneous letter in a gene called TMC1 that causes the animals to gradually lose their hearing. The genetic defect affects the function of small hair cells in the inner ear that detect acoustic vibrations. As time goes on, most of the hair cells die off in mice — and people — with the genetic mutation.
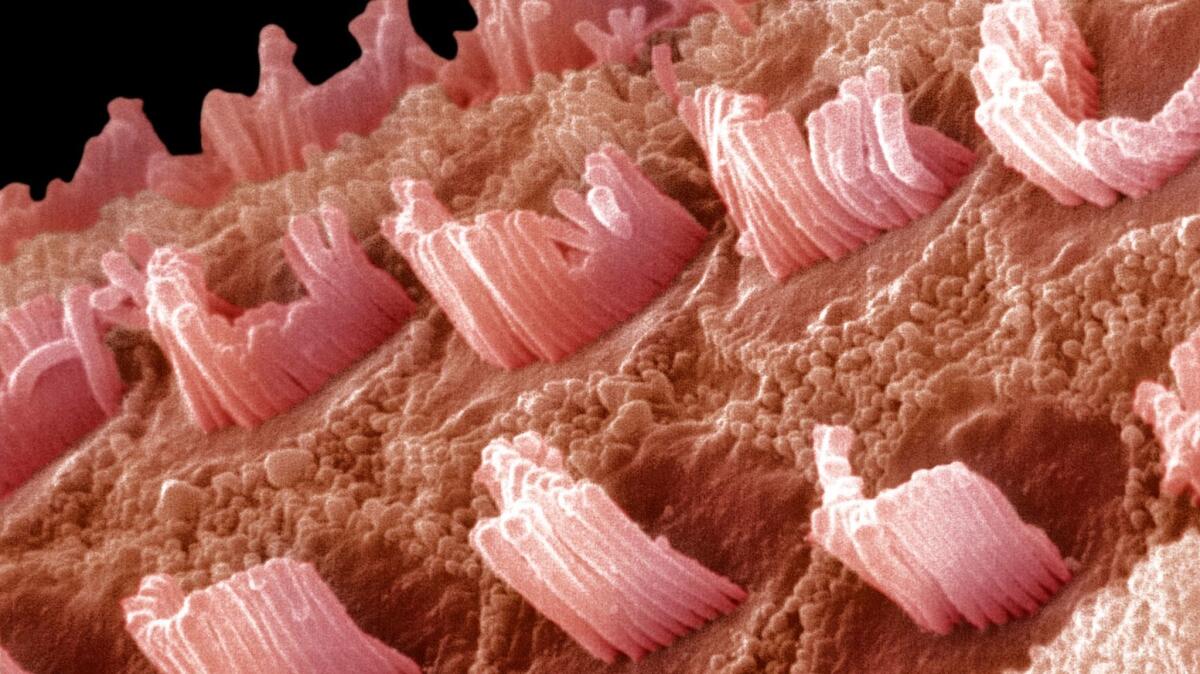
To keep this from happening, the authors set out to use the CRISPR gene editing system to bind to and cut the faulty TMC1 gene, allowing the good copy to become dominant.
Xue Gao, a post-doctoral researcher in Liu’s lab and the first author on the paper, faced two main challenges when she took on this work, Liu said. She had to figure out the best way to get the CRISPR system into the hair cells of a mouse’s inner ear. She also had to make sure that the CRISPR complex disrupted only the bad copy of TMC1 and left the good copy intact.
To get the CRISPR machinery into the cells, Gao turned to a method developed by Liu’s lab in 2015 of using tiny lipid bubbles to encapsulate the CRISPR complex. The bubbles — Liu describes them as minuscule soap bubbles — are positively charged which allows them to bind with the cell membrane fairly easily.
Ensuring that the CRISPR system only altered the bad copy of TMC1 proved a little more challenging, however.
In Beethoven mice, and in humans with the same genetic defect, the two copies of the TMC1 gene differ by only one letter, or nucleotide. That makes the good copy an easy target for the gene editor.
Indeed, in early experiments in test tubes, the authors found that their CRISPR system didn’t do a good job of picking out the bad gene over the good one.
“We were tempted to give up at that point,” Liu said. “If you don’t have a selective editing strategy, then you wind up damaging the healthy form of the gene. That would lead to hearing loss rather than preservation.”
Once again, a previous study came to the rescue.
Scientists often insert instructions for building the CRISPR complex into cells, rather than inserting the complex itself, Liu explained. However, research has shown that if you deliver a pre-assembled CRISPR system into the cell, it tends to have less off-target editing errors.
“That gave us hope,” Liu said.
When Gao tried the experiment with the pre-made CRISPR gene editor, the number of off-target edits dropped significantly.
Once these two hurdles were cleared, the team injected a cocktail of CRISPR and lipid bubbles into the inner ears of Beethoven mice and then tested the effects of the treatment.
After eight weeks they found that the number of hair cells in treated Beethoven mice were almost indistinguishable from those in mice without the genetic mutation. Meanwhile, the inner ears of untreated Beethoven mice were almost completely devoid of the hair cells.
The researchers also ran the mice through a suite of hearing tests. These showed unequivocally that Beethoven mice that had received the CRISPR-lipid cocktail had indeed retained more of their hearing than those that did not.
Fyodor Urnov, a molecular biologist at the Altius Institute for Biomedical Sciences in Seattle who was not involved in the work, said the new study provides an essential first step toward moving this type of approach nearer to the clinic.
But in an essay accompanying the study, he cautioned that several more steps will need to be taken before we get there.
For starters, Liu said this method will need to be tested in larger animals before clinical trials in humans can be considered.
“Mice have been cured of many diseases,” he said.
Do you love science? I do! Follow me @DeborahNetburn and “like” Los Angeles Times Science & Health on Facebook.
MORE IN SCIENCE







