Op-Ed: How to pay the bill for hepatitis C

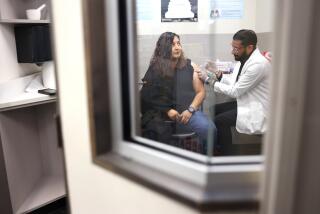
Harvoni, a pill from Gilead Sciences, accounted for more than three-fourths of the prescriptions filled for hepatitis-C drugs in the first three months of this year, according to IMS Health. Above, the headquarters of Gilead Sciences in Foster City, Calif.
How long should you wait to treat a possibly fatal but curable disease?
That’s a question with major implications for millions of patients and for insurers and government programs that have to pay for the treatment.
In the last year this question has focused on hepatitis C, a viral infection of the liver that, left untreated, can lead to cirrhosis, cancer, liver failure and death. Hepatitis C is the leading cause for liver transplants in the United States.
The virus can be eliminated in more than 90% of the individuals who take newly approved drugs over a three-month treatment — but at a staggering cost of $75,000 to $100,000 per patient. Treating everyone in the U.S. who has the hepatitis C virus would cost at least $200 billion. To put that in perspective: In a typical year, U.S. spending on all prescription drugs is $300 billion to $360 billion.
In July the California Department of Health Care Services ordered a new protocol that will mean many thousands of Medi-Cal patients will have to wait for treatment. But far from jeopardizing lives, the department is helping to lead the way out of the hepatitis C conundrum with a sensible policy: Treat everyone who needs it, but not until treatment is necessary.
As serious as hepatitis C can be, it often produces no symptoms — no cirrhosis, cancer or liver failure. Many of the estimated 3 million to 7 million infected people in the U.S. and 130 million to 170 million worldwide do not know they have the disease; most fail to develop symptoms over their lifetime. Statistically, only a third will ever be seriously troubled by their infection.
The California protocol essentially calls for two things: watchful waiting, and using simple screening indicators. One indicator — called FIB-4 — is calculated from routine blood tests and estimates the amount of scarring on the liver. It is inexpensive, easy to measure and shown to be highly predictive of the disease’s progression.
Treatment to eliminate the virus can be delayed until the marker shows heightened liver fibrosis (scarring), without significantly increasing a patient’s risk for complications, illness and death.
The marker was revealed during research we began four years ago with pharmaceutical companies and the Veterans Administration aimed at demonstrating to infected veterans when they needed to embark on a different hepatitis C treatment — an onerous, yearlong drug regimen — or face organ failure. Now that same marker can tell how long new, more effective drug treatments developed by other companies can be safely delayed for patients with minimal symptoms or merely a diagnosis of hepatitis C. A simple test every six months or so can alert a physician to whether the risk of serious complications is increasing.
The California protocol will, in a way, ration care, and any form of rationing is controversial. Consumer groups and some health insurance companies have called for investigations into the costs of hepatitis C drugs to pressure drug makers to roll back prices. Manufacturers contend their products are a bargain, even at a very high price, because the American healthcare system would otherwise have to deal with the long-term consequences of millions of chronic hepatitis C cases. (The first of these medications are already coming under price pressure after the introduction of competing therapies.)
But the state’s protocol provides a way through the thicket. A California Assn. of Health Plans study released in June estimated that treating the 127,000 Medi-Cal patients with hepatitis C for one year might cost $438 million to $1.71 billion. By implementing the screening protocol, Medi-Cal can probably hold costs to the lower part of that range without risking patient health.
Other states are starting to implement similar protocols. Now physicians, health insurance companies, managed care organizations and other government healthcare programs need to follow suit to manage the new, highly effective but very expensive treatments for hepatitis C.
Doing so will strike a practical, and humane, balance. Knowing who does not need treatment right away improves access for those who do.
D. Steven Fox, an assistant professor at the Keck School of Medicine at USC, and Jeffrey S. McCombs, an associate professor at the USC School of Pharmacy, are affiliated with USC’s Leonard D. Schaeffer Center for Health Policy and Economics. Neither has a financial interest in hepatitis C therapies or tests.
Follow the Opinion section on Twitter @latimesopinion and Facebook
More to Read
A cure for the common opinion
Get thought-provoking perspectives with our weekly newsletter.
You may occasionally receive promotional content from the Los Angeles Times.