Bankruptcy is ‘an option’ for OxyContin maker Purdue Pharma, CEO says
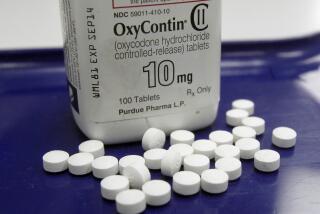
Purdue Pharma’s chief executive said the company is considering bankruptcy as it faces a cascade of lawsuits alleging that the drugmaker played a key role in driving the nation’s opioid crisis, including aggressively and deceptively marketing the powerful painkiller OxyContin.
Craig Landau, Purdue’s president and CEO, said his company has not yet decided whether to file for bankruptcy protection, but he said the company is weighing doing so as it considers the impact of potential legal settlements or jury verdicts that could cost tens of billions of dollars.
“It is an option,” Landau said. “We are considering it, but we’ve really made no decisions on what course of actions to pursue. A lot depends on what unfolds in the weeks and months ahead.”
Purdue has denied the allegations lodged against it in court and is mounting a vigorous defense. Most of the lawsuits are consolidated in a federal court in Cleveland, where settlement talks have been ongoing for more than a year as lawyers simultaneously prepare for two trials scheduled for the fall.
Declaring bankruptcy could halt litigation against the company, bankruptcy lawyers said, and it can be more difficult for plaintiffs to secure judgments in Bankruptcy Court than in civil court.
Purdue is one of a number of defendants in a separate Oklahoma state trial scheduled to begin May 28. It will be the first trial at which a jury could decide whether drug companies bear responsibility for the opioid crisis; settlement talks are ongoing. People familiar with the case have said Oklahoma could seek more than $1 billion from the defendants.
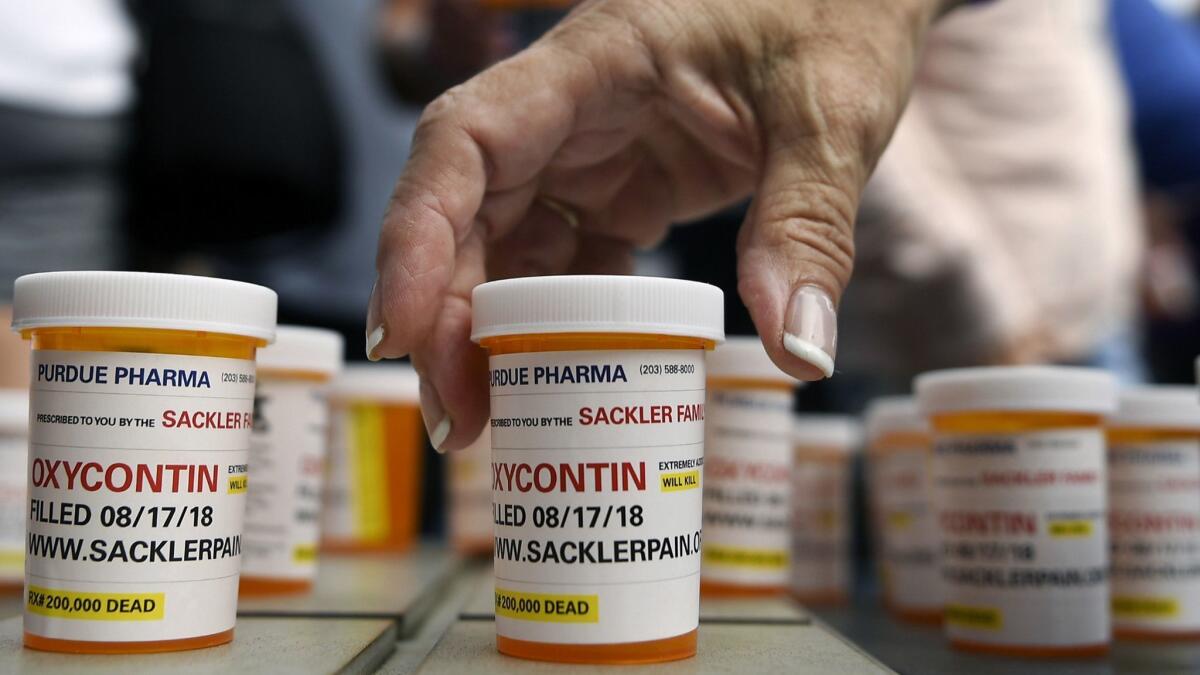
Purdue also is facing a lawsuit in Massachusetts that has made public numerous internal documents that the state attorney general argues are evidence the company disregarded safety and addiction as it pursued massive profits from patients who became dependent on its products. Purdue said the lawsuit amounts to “oversimplified scapegoating” that is not supported by the law.
Landau declined to discuss the pending litigation. Purdue has argued that it controls just 2% to 3% of the market for opioid drugs. It also says it is not responsible for overdose deaths from heroin and illicit fentanyl, a powerful synthetic opioid made overseas that has killed tens of thousands of people in recent years and is now the leading driver of overdose deaths.
Purdue announced Wednesday that it is developing a drug to reverse opioid overdoses that it claims is far more powerful than naloxone, the well-known opioid overdose antidote currently in use across the country.
The company said the Food and Drug Administration has granted the drug — an injection of nalmefene hydrochloride — the agency’s fast-track designation, which will speed up its pharmaceutical development and agency review. The FDA fast-tracked over-the-counter naloxone in January, and it is moving quickly on non-opioid drugs to treat pain.
The FDA said Tuesday that it could not comment on the designation until Purdue announces it. The drug needs FDA approval before it can reach consumers.
Landau said Purdue is making the drug as a pro bono effort to stem overdose fatalities.
“We’re not looking to make money, nor do we intend to profit in any way from the development and availability of this hopefully lifesaving treatment,” he said.
Landau said the drug has been in the pipeline for about two years and is intended to serve as an antidote to fentanyl overdoses; first responders have said numerous doses of naloxone are needed to reverse some fentanyl overdoses because fentanyl is so potent.
Despite the fast-track designation, it probably will take years for the new drug to reach consumers. Landau said the goal is to get it to people who need it as soon as possible. No other antidote of its strength is currently available in the United States, he said.
Fentanyl is the third wave of the opioid epidemic, which started with prescription painkillers. After the government cracked down on illicit prescribers and tried to address the flow of pills to the black market, those addicted to the painkillers turned to heroin.
Landau disputes claims that Purdue and its OxyContin pills were responsible for sparking the opioid crisis.
“I don’t believe one company or one product was the cause of the opioid crisis. I and many others understand the opioid crisis to be very complicated, with many contributing factors, but this program that we’re discussing is very simple,” Landau said. “The motivation has really nothing to do with who did or who didn’t cause an opioid crisis or litigation. It’s really meant to save lives.”
Purdue has launched numerous programs to combat opioid abuse. Several came after the company paid $600 million in fines in 2007 as part of a federal case. Company executives also pleaded guilty to criminal charges after claiming the product was not as addictive as other opioids.
The company’s initiatives include providing millions of dollars to support the creation of an over-the-counter naloxone spray and training law enforcement to administer the drug; supporting education on the proper storage and disposal of prescription opioids; and conducting research into non-opioid painkillers.
Landau said creating the overdose antidote is another way Purdue is working to fight the opioid crisis.
“We believe we have a responsibility as a pharmaceutical company to do what we can, and we’re leaning forward with what we believe could be a very meaningful solution and a very meaningful contribution to the public health,” Landau said.
Landau said he’s sure his company, after being accused of sparking the opioid crisis, will be criticized for attempting to help solve it.
“I recognize everything we do will be criticized in this regard, but in the end we’re going to do what’s right,” he said. “These are good things that could and should have a positive impact on public health and on patients.”
More to Read
Inside the business of entertainment
The Wide Shot brings you news, analysis and insights on everything from streaming wars to production — and what it all means for the future.
You may occasionally receive promotional content from the Los Angeles Times.