Hope for spinal cord patients
History was made this month when a patient in Atlanta was treated for the first time with a therapy derived from human embryonic stem cells. It was part of a clinical trial to test the safety of specialized nerve cells that researchers hope will repair damaged spinal cords — potentially allowing patients to regain the use of their legs, bladders or even just a single finger that would allow them to operate their own wheelchair.
There is currently no cure for the paralysis that results from a traumatic spinal cord injury. And while the world watches this trial, researchers say no one should expect it to produce a miracle cure.
“There’s so much hype about stem cells,” says Dr. Michael Sofroniew, a neurobiologist at UCLA’s David Geffen School of Medicine. “Yes, there’s enormous potential there, but we have to approach it in a realistic way.”
An estimated 262,000 Americans are living with some degree of paralysis as the result of a spinal cord injury. An additional 10,000 to 12,000 suffer irreparable damage to their spinal cords — most commonly due to car crashes, but also from violent attacks, falls and sporting accidents — each year, according to the National Spinal Cord Injury Statistical Center.
Here’s a closer look at how human embryonic stem cells are being put to use to treat spinal cord injuries and other debilitating conditions.
What is spinal cord injury?
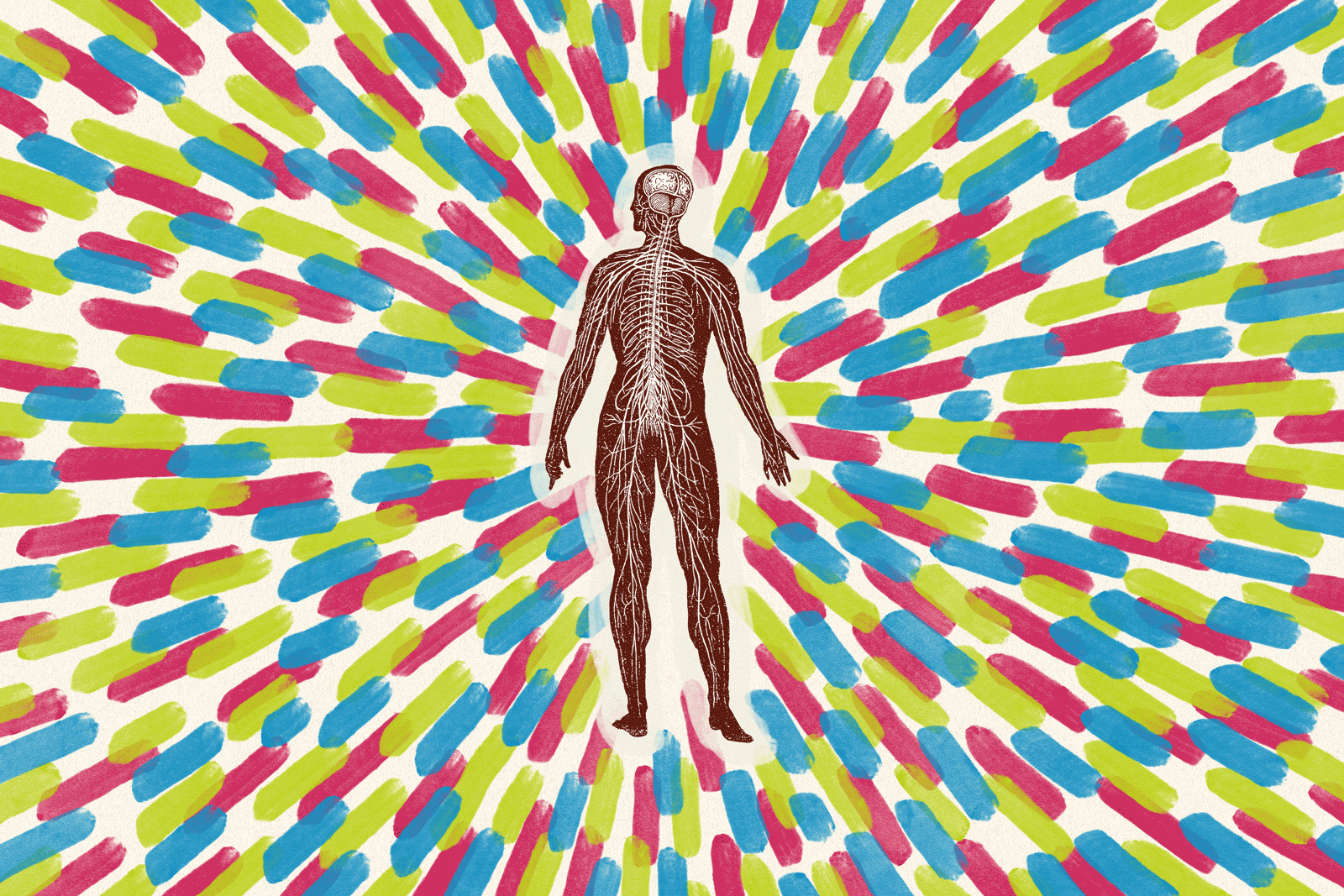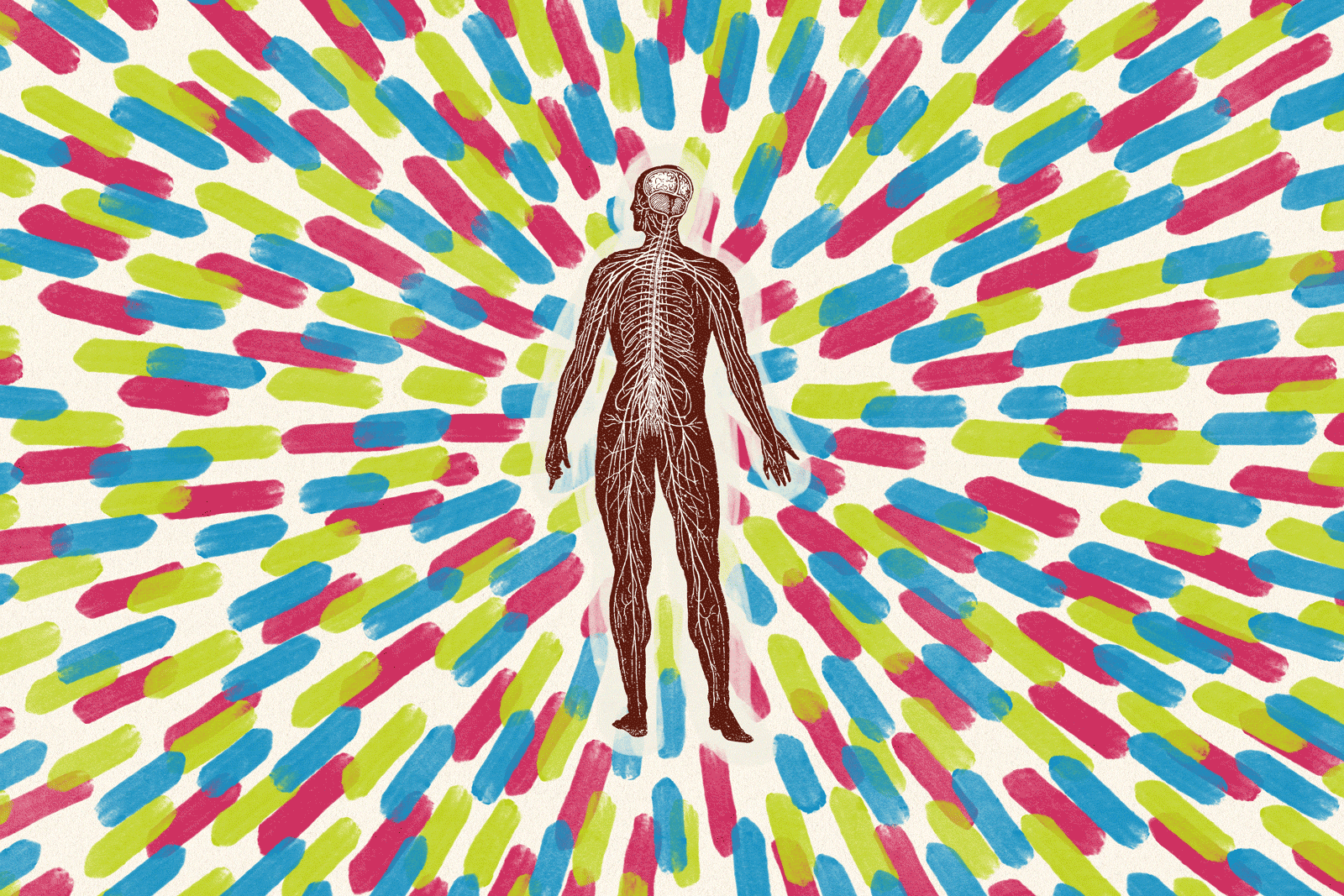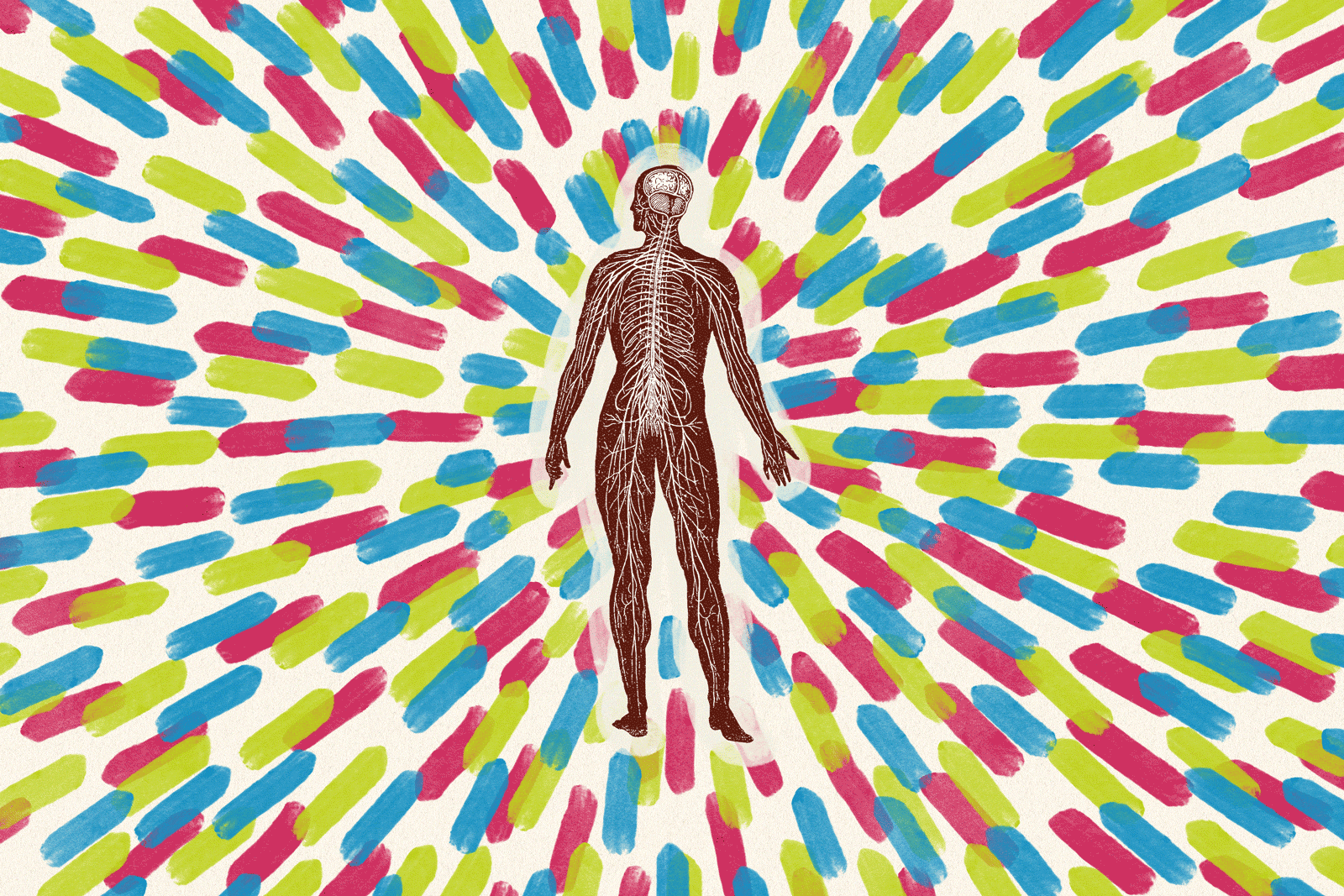
The spinal cord is the major highway for nerve cells that relay information between the body and the brain. The cord is protected by the vertebrae of the spine, but a blow to the back or neck can cause the cord to bruise or tear. How much function a person loses depends on which part of the cord is affected, along with how many and which nerve fibers are damaged.
The original trauma to the nerve cells can strip them of their insulating sheath of fat and protein called myelin. Without myelin, a nerve’s ability to conduct an electrical impulse is severely impaired. “It’s the difference between being able to transmit and not being able to transmit,” says Hans Keirstead, the UC Irvine neuroscientist who invented the embryonic stem cell therapy now being tested in Atlanta by Geron Corp. of Menlo Park, Calif.
Over time, a secondary degeneration occurs, in which damaged fibers slowly die. The stem-cell therapy addresses both of these processes, Keirstead says.
How are human embryonic stems cells used as therapy?
The cells are grown in the laboratory and treated according to a specific biochemical regimen that encourages them to develop into a kind of nerve cell called an oligodendrocyte progenitor cell. These cells help an injured spinal cord in two ways: They restore the necessary myelin, and they release loads of growth factors that nurse the tissue and prevent secondary loss of neurons.
In experiments with rats, the oligodendrocyte progenitor cells improved the animals’ ability to walk, and even run with a limp, after injuries to the thoracic (midbody) and the cervical (neck) spinal cord. The researchers also found that more myelin and more neurons were saved in treated animals. Keirstead’s team published these results in a 2005 Journal of Neuroscience study and a 2010 paper in the journal Stem Cells.
Who is eligible to enroll in the Geron trial?
For now, scientists are focusing on injuries that are no more than two weeks old and have the best odds of being reversed with proper treatment. But they don’t want patients whose injuries are less than one week old because the body’s natural healing process could damage the therapeutic cells.
In addition, the clinical trial is testing only thoracic injuries, because if the cells don’t work — or worse, cause harm — the outcome would be less severe than with cervical injuries. “Close to your neck is your brain stem, which controls your heart rate, your breathing and your temperature regulation,” Keirstead says. “You mess with that and you die.”
Why use embryonic stem cells instead of other types of cells?
Theoretically, scientists could try to create a cell-based therapy that starts with adult stem cells or fetal-derived stem cells, or they could attempt to grow mature oligodendrocytes in the lab. However, Keirstead says, “you can’t get enough, and then the ones that you get don’t divide well.” Embryonic stem cells, on the other hand, divide like crazy and can be used to make enough of the therapy to treat a patient.
How are spinal cord injuries usually treated?
The standard treatment usually includes surgery to decompress the injury site and stabilize the spinal cord, says Dr. Robert Grossman, chairman of neurosurgery at Methodist Hospital in Houston, who is leading a clinical trial of a neuroprotective drug called riluzole for the New Jersey-based Christopher and Dana Reeve Foundation.
In addition, a variety of treatments are aimed at addressing the many things that go wrong after a spinal cord injury. For example, drugs and hypothermic treatment administered early on may help protect against neuron loss and control inflammation.
Following that, patients receive sophisticated exercise programs to try to regain lost mobility. Treatments that trigger small improvements in neuron growth may be enhanced by training regimens. “There’s more capacity for plasticity and reorganization in the nervous system than was appreciated 20 years ago,” UCLA’s Sofroniew says.
Other experimental treatments are also being developed to stimulate regeneration of myelin and nerve connections. For example, an antibody called anti-Nogo was found to be safe in a clinical trial. The next step is to test its effectiveness, according to a review paper published this year in Annals of the New York Academy of Sciences.
How will the success of the stem-cell trial be measured?
This is a safety study that will enroll no more than 10 patients and follow their neurological condition for one year. In addition, researchers will be watching closely to see whether the cell-based therapy causes tumors or pain. Tumors can result from transplants containing undifferentiated stem cells, which have been purified out of the Geron product, but it’s still a theoretical risk, Keirstead says. Pain could occur if the oligodendrocyte progenitor cells trigger the branching of pain fibers within the spinal cord.
If safety is confirmed, Geron would proceed with a Phase 2 trial designed to gauge the cells’ effectiveness in repairing spinal cord injuries.
What other diseases might benefit from embryonic stem cells?
At least one other company has asked the U.S. Food and Drug Administration for permission to proceed with clinical trials of a therapy derived from human embryonic stem cells. Santa Monica-based Advanced Cell Technology Inc. wants to test its lab-grown retinal pigment epithelium cells in patients with a rare eye disorder called Stargardt’s macular dystrophy, a childhood version of macular degeneration.
In the long run, embryonic stem cells could be used to develop treatments for other neurodegenerative diseases, such as Parkinson’s disease and multiple sclerosis. “We’re embarking on a completely new approach to medicine,” Sofroniew says.