Op-Ed: California has gone crazy for sketchy stem cell treatments
In case you haven’t noticed, stem cell clinics are popping up everywhere. There are hundreds across the country, especially in California. The clinics peddle “vegan stem cell facials” or “stem cell vaginal rejuvenations” and claim the miracle cells can treat autism, baldness, dementia, diabetes, arthritis and paralysis all with a quick injection.
If it sounds too good to be true, it is.
For the record:
1:40 p.m. Jan. 25, 2019 Californians passed Proposition 71 in 2004, not 2014.
There is no good scientific evidence the pricey treatments work, and there is growing evidence that some are dangerous, causing blindness, tumors and paralysis. Medical associations, the federal government and even Consumer Reports have all issued stern warnings to patients about the clinics.
So why do patients keep streaming in for treatments that cost thousands of dollars? Part of the reason, I suspect, is that stem cell research — the serious, scientific kind — has gotten so much hype in recent years. We’ve all heard about how some stem cells have the power to become any type of cell in the body and might one day offer cures for all manner of crippling and degenerative diseases. If you can jump the line, and get those treatments now, why not do it?
California is the state with the most stem cell clinics in the country offering these unproven “cures.”
Here’s why: Because the days of miraculous cures, if they come, are far in the future. Today, there is only one federally approved stem cell product: the limited use of blood-forming stem cells to treat certain blood disorders. Scientists are just beginning to learn how to harness the power of stem cells, and the harsh reality is that clinical trials that could turn that knowledge into effective therapies will take years, if not decades.
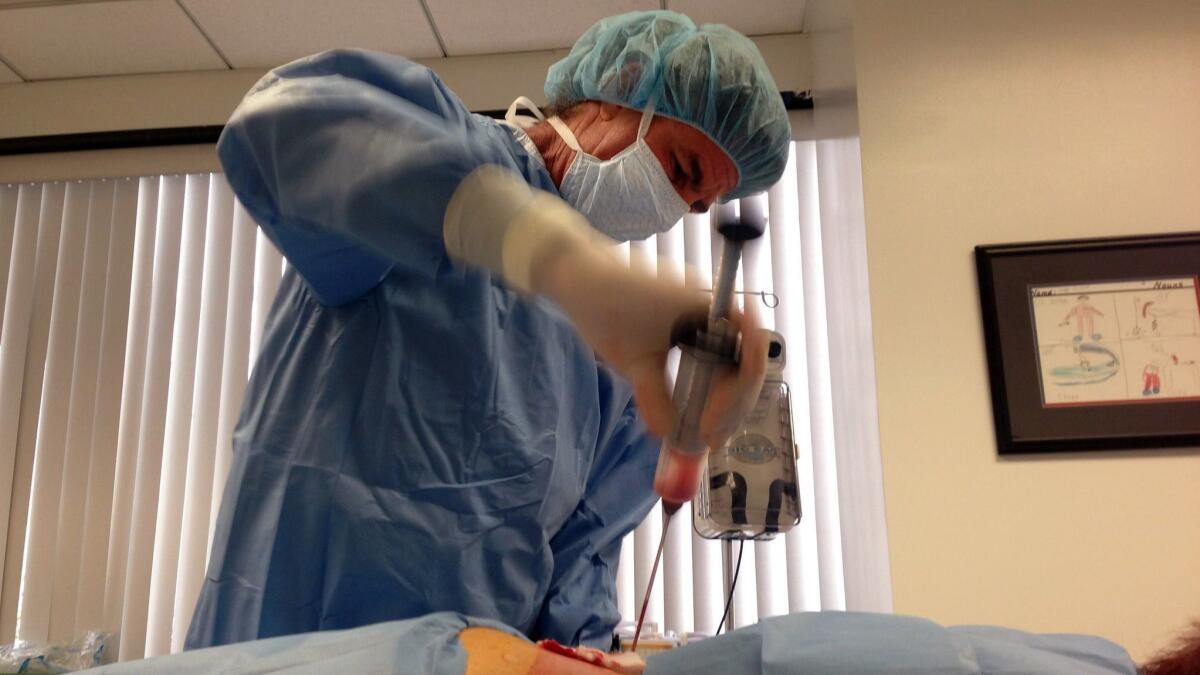
Entrepreneurial physicians surely know this, but it hasn’t stopped them from piggybacking on the hype and excitement, injecting stem cells they pull from patients’ own abdominal fat or blood, into joints, bloodstreams, spinal cords and brains with abandon and often charging thousands of dollars per treatment.
The U.S. Food and Drug Administration has so far moved cautiously, in part because the cells fall into a regulatory gray zone. Biologic materials like organs, tissue and blood are regulated to ensure that material that is processed or moves from one person to another won’t cause infection or other problems.
But clinic operators say normal rules don’t apply to them since most of their treatments are barely processed and use a patient’s own cells. They argue that patients have the right to use their cells and cite anecdotal evidence that the treatments work. Some clinic operators say they believe in the treatments so strongly, they routinely inject themselves.
The regulatory void leaves patients vulnerable to outright fraud. Patients often have no way of knowing whether a syringe even holds live stem cells. In some cases where stem cell products have been inspected, they have been found to contain only dead stem cells, or things you wouldn’t want to be injected with, like the traces of fecal bacteria that recently left a dozen patients hospitalized.
And even if live stem cells are injected into the body or bloodstream, there is absolutely no evidence they’ll migrate where they need to go and start repairing damaged organs and tissues. They are cells, not magic. And they can be hard to keep alive in ideal laboratory conditions. Injected into a patient and out of biological context, they probably just die.
California is in an odd position. It is the state with the most stem cell clinics in the country offering these unproven “cures.” It also happens to be a world center of serious scientific stem cell research, thanks to a $3-billion ballot initiative, Proposition 71, passed by voters in 2014 to fund research.
In the absence of clear federal regulation, the state has tried to step in to combat rogue clinics — but not with the kind of muscle it so famously used with car emissions. While California was the first state to pass a law requiring stem cell clinics to post a notice that their therapies are not FDA approved, the law is weak. And it’s not clear whether it is being enforced or whether the notices sway patients.
Enter the Fray: First takes on the news of the minute »
The California Medical Board, which has the power to revoke the licenses of physicians, is currently eyeing the issue of stem cell clinics with a task force and may take action. But the situation also demands broad action from the U.S. Food and Drug Administration, which has moved inexplicably slowly and dodged the issue for years.
There may finally be a glimmer of hope, however. FDA Commissioner Scott Gottlieb has earned kudos for taking a stronger public stance on stem cell clinics than any of his predecessors. He’s taken action against a few of the most unsafe clinics and has issued a sternly written public warning to consumers. The FDA is also now in court seeking a permanent injunction against two clinic groups. The agency has promised to regulate the clinics more strictly by the end of 2020. But that’s far too much time to let unproven stem cell clinics stay in business.
Here’s an idea in the meantime. The many scientists who have benefited from taxpayer support of stem cell research in the state should start speaking out. After all, the hype from proponents of Prop. 71 is part of what created such high expectations for quick cures – and eagerness on the part of patients to get them. Scientists should now take every opportunity both to explain to the public the long-term goals of their research and the absurdity of the so-called cures now flooding the market.
Usha Lee McFarling was a science writer at the Los Angeles Times from 2000 to 2006. She won a Pulitzer Prize in explanatory journalism in 2007.
More to Read
A cure for the common opinion
Get thought-provoking perspectives with our weekly newsletter.
You may occasionally receive promotional content from the Los Angeles Times.