All donor blood in the U.S. should be screened for Zika, FDA says
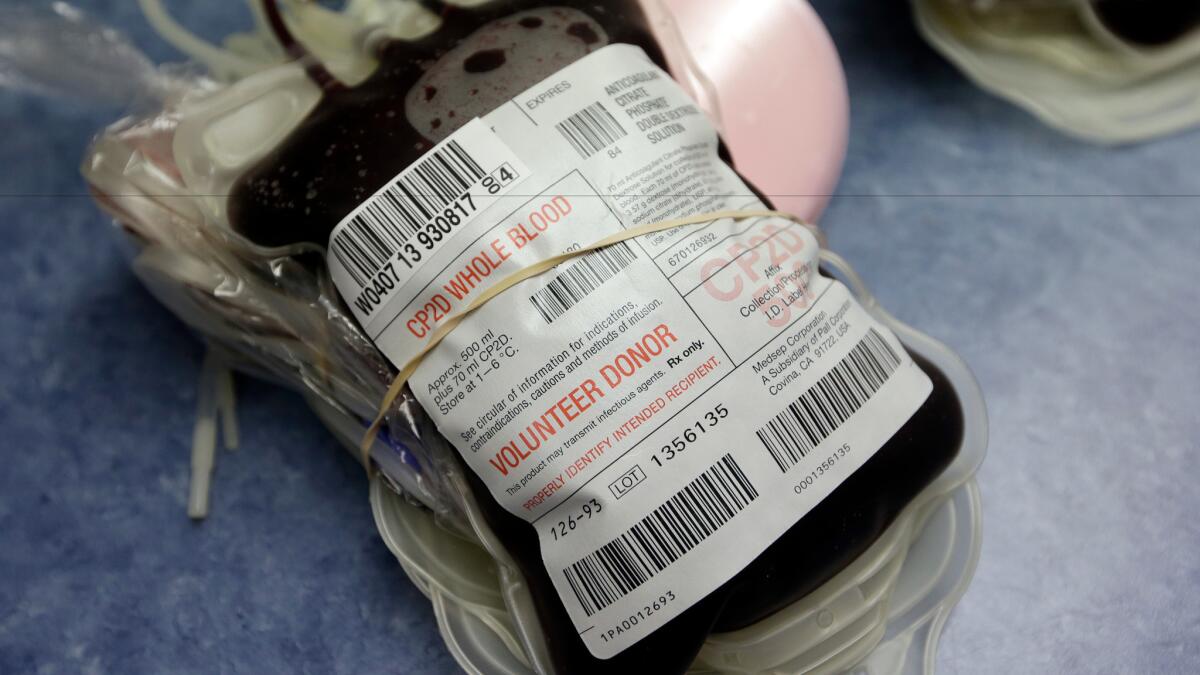
All blood donated in the U.S. should be screened for Zika to prevent the virus from spreading through transfusions, the Food and Drug Administration said Friday.
The new guidance should be implemented “immediately” in states and territories where the virus is already being spread by mosquitoes, and it should be phased in over the next four to 12 weeks in the rest of the country.
“The recommendation for testing the entire blood supply will help ensure that safe blood is available for all individuals who might need transfusion,” Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, said in a statement.
Zika is spreading rapidly throughout the Americas, with 50 countries and territories now dealing with active outbreaks.
As of Wednesday, 8,746 people in Puerto Rico have been infected with the virus locally, along with dozens of additional cases of local transmission in the U.S. Virgin Islands and American Samoa, according to the Centers for Disease Control and Prevention.
Zika is a mosquito-borne illness that has spread through dozens of countries in Latin America, the Caribbean, and now the United States.
Florida is the only U.S. state with Zika infections that can’t be linked to travel. As of Wednesday, 29 people there have been diagnosed with laboratory-confirmed infections, the CDC says.
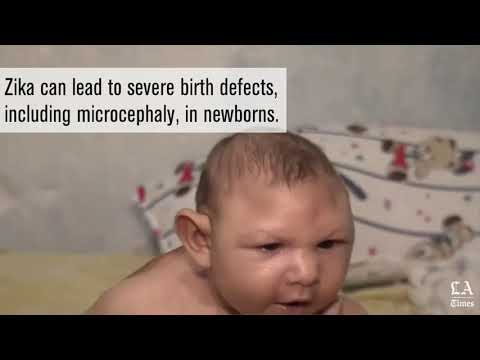
The Zika virus is spread by mosquitoes through their bites. Once infected, a person can spread it to another through sexual contact. Most notably, an infected pregnant woman can pass the virus to her unborn child, putting the baby at risk for microcephaly and other brain-related birth defects.
Donor blood can be another important source of Zika transmission. During a Zika outbreak in French Polynesia in 2013 and 2014, nearly 3% of blood samples from people with no sign of infection were found to contain the virus, which could have been spread to others through routine infusions.
And in Puerto Rico, where screening has been recommended since February, nearly 1% of blood samples from donors with no symptoms of Zika turned up positive for the virus, according to the FDA.
Screening potential donors before they give blood is unreliable, since four out of five infected people never develop any outward sign of infection. Among those who do, most have vague symptoms like fever, headaches or joint or muscle pain.
To get around this problem, the FDA’s new guidance calls for testing all donated blood using a so-called nucleic acid test. These tests search for specific genetic sequences in certain viruses, such as HIV or hepatitis. Versions that look for Zika are still undergoing final FDA review.
If any blood is found to be infected, any other blood given by the same donor in the past 120 days should be quarantined, according to the new guidelines. If some of that blood has already been used to treat another patient, the recipient’s doctor should be notified.
If no screening test is available, blood collection agencies can purify blood platelets or plasma using one of the FDA’s approved methods. In the future, if a pathogen reduction technology becomes available for red blood cells or for whole blood, these methods could be used instead of a nucleic acid test, the FDA said.
Although there’s no longer any need to ask potential donors about their recent travel to regions affected by Zika, any prospective blood donor who says they have been infected with Zika should be asked to wait until their symptoms have resolved or for 120 days, whichever is longer, the FDA said.
The new guidance applies immediately in Florida and in territories with at least one case of a locally acquired infection. All blood collection should be put on hold until the new procedures can be implemented, the FDA advises.
Blood collection agencies in states that have reported travel-related Zika infections or that are close to areas where mosquitoes are known to have the virus should implement the guidelines within the next four weeks. These include Alabama, Arizona, California, Georgia, Hawaii, Louisiana, Mississippi, New Mexico, New York, South Carolina and Texas.
Facilities in all other states should be following the new guidelines within the next 12 weeks, the FDA says.
Follow me on Twitter @LATkarenkaplan and “like” Los Angeles Times Science & Health on Facebook.
MORE IN SCIENCE
Meet Octobot, a soft-bodied robot that moves like an octopus
The aging paradox: The older we get, the happier we are
Your coffee habit may be written in your DNA