Column: With most drug ingredients coming from China, FDA says shortages have begun
As consumers worldwide adapt to life under the coronavirus, George Timek had what he thought was a perfectly reasonable question: Where is his medicine made?
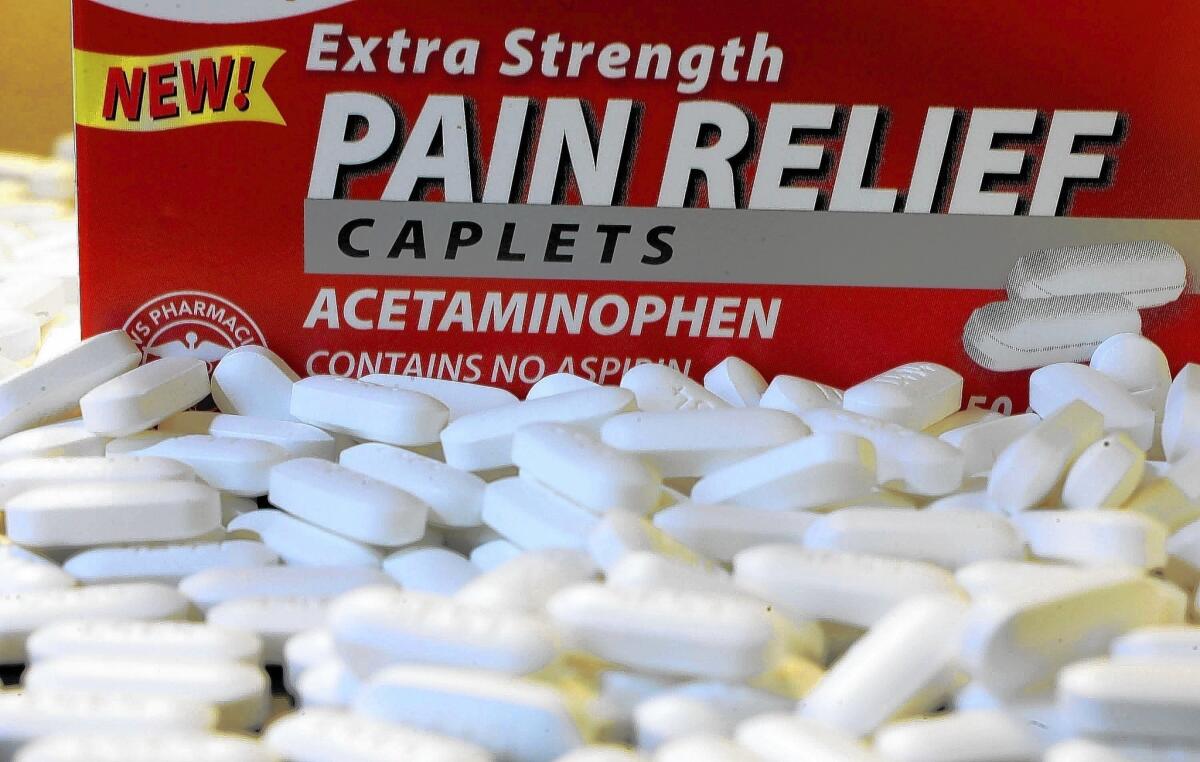
We’re not talking about anything fancy. Timek, 76, was shopping for acetaminophen at BJ’s Wholesale Club and was curious about the country of origin.
He looked and looked. Nowhere did it say where the product was manufactured.
“Is this legal not showing country of origin on the bottle?” he asked me.
The answer is yes, although country of origin does have to be disclosed somewhere, possibly on the outer box, possibly online.
Be that as it may, tracking down what many people — especially now — might view as a crucial piece of information is much harder than it should be.
Timek’s concern also highlights a challenge on the horizon for millions of Americans: maintaining healthcare regimens amid disruptions to the global supply chain.
Your medicine, prescription or otherwise, may not come from China (although many do), but there’s a high likelihood it contains ingredients that originated in China and other countries affected by the coronavirus.
Stephen Hahn, commissioner of the Food and Drug Administration, said Friday that the coronavirus has resulted in at least one drug shortage because an active ingredient is unavailable.
He didn’t identify the drug or the ingredient, saying only that “this shortage is related to a site affected by coronavirus.”
Experts warn this may be just the beginning.
“One of the ugly secrets of the pharmaceutical industry is that the vast majority of raw materials that go into a prescription drug are produced overseas, mostly in China and India,” said Geoffrey Joyce, chairman of the Department of Pharmaceutical and Health Economics at the USC School of Pharmacy.
“The coronavirus shutting down China or India for an extended period of time is likely to have a substantial impact on the supply of many drugs,” he said.
William Comanor, a professor of health policy and management at UCLA, was even more emphatic.
“Tell your readers to stock up on generics!” he said. “You’re going to see shortages.”
That may be just a tad alarmist. But I’ll admit I’ve ordered additional supplies of my own meds.
BJ’s is an East Coast big-box discounter that competes with Costco. Like many retailers, the company has its own in-house brands that serve as cheaper alternatives to the big national brands.
Its Berkley Jensen acetaminophen is a generic rival to Tylenol.
Nowhere does it say on the BJ’s website where the product comes from. A BJ’s service rep told me it’s manufactured in the United States.
What about the ingredients?
“I don’t know,” the rep answered after tapping away at her computer keys. “It doesn’t say.”
She said BJ’s acetaminophen, like many of the company’s over-the-counter drugs, is produced by a company called Perrigo, which is based in Michigan but, for tax purposes, claims Dublin, Ireland, as its official headquarters.
Perrigo says its products “are available at retailers, pharmacies and e-commerce outlets in the United States, across Europe and in other major markets.”
Before we go any further, it’s important to note that U.S. Customs law has a big loophole when it comes to country-of-origin labeling.
It doesn’t require that the sources of a drug’s ingredients be disclosed. Rather, the law says a drugmaker can claim as the country of origin wherever the drug’s various components were “substantially transformed” into the final product.
That means a drug manufacturer can gather ingredients from around the world. But if it pulls them all together into pill form in the United States, the country of origin can be claimed as the U.S.
This, of course, does consumers no favors.
UCLA’s Comanor observed that the vast majority of U.S. drug sales involve generics, so coronavirus-related disruptions may have a lesser impact on name-brand pharmaceutical companies, which favor domestic production of ingredients.
But for everything from generic over-the-counter drugs to generic prescription meds, he said, consumers are at the mercy of global supply chains.
“I want to be able to buy generics made from active ingredients produced in the U.S.,” Comanor said. “I cannot do that.”
The generic acetaminophen that Timek purchased is a good case in point.
The country of origin — that is, where the final tinkering was performed — may be the United States, but the ingredients are more like an episode of “Dora the Explorer.”
Acetaminophen, also known as paracetamol, is America’s most widely used drug ingredient, found not just in namesake pills but also as a component of hundreds of cold, flu and allergy remedies.
A recent study determined that 84% of the world’s supply of acetaminophen comes from China and India, with China accounting for nearly two-thirds of the total.
The United States is the world’s single largest market for generic acetaminophen, the study found.
Then there are all the inactive ingredients in the BJ’s acetaminophen. These include various components that go into making a pill a pill, such as croscarmellose sodium, crospovidone and assorted colorings, all of which almost certainly come from overseas factories, primarily in China.
I reached out to both Perrigo and BJ’s for comment. Neither company responded.
Pharmaceutical experts emphasized that most drug factories worldwide operate under strict safety standards.
Just because an ingredient comes from China or India doesn’t mean it’s unsafe. But it does mean that if factories in those countries face production difficulties, shortages of finished drugs can be felt around the planet.
At this point, it’s unclear how widespread the disruptions are. Large U.S. drug companies say they have sufficient inventory to handle demand, at least in the near term.
The FDA says it has contacted dozens of Chinese manufacturers with a reminder that they have to report any disruptions. It calls the situation “evolving and very dynamic.”
Experts say it would be unlikely for overseas companies to stockpile most raw ingredients solely for domestic use. But some fear China could curtail exports of materials used in the production of antibiotics if demand for such drugs surges at Chinese hospitals.
About 90% of the core components of leading antibiotics such as amoxicillin and penicillin come from China.
“You could say that when China sneezes, the average American consumer feels it, and this is because of the nature of the global supply chain,” said Adegoke Oke, an associate professor of supply chain management at Arizona State University.
“Consumers must get used to the fact that their meds, and in fact many other products, come from all over or depend on ingredients from all over,” he said.
Rachna Shah, an associate professor of supply chain and operations at the University of Minnesota, said the FDA does a reasonably good job of monitoring the safety of drug ingredients produced worldwide.
“However, there is no central mechanism to ensure drug availability,” she said. “Disruptions such as the coronavirus expose this dependence on foreign sources and heighten the fears associated with drug shortages.”
She and others said the drug industry is scrambling to keep shelves stocked.
“Companies have business continuity plans and I am sure are working hard to respond,” said Ravi Anupindi, a professor of operations research and management at the University of Michigan.
He added, however: “They need to reduce their dependence on China.”
I agree. I’d also say it’s time that the FDA cast more sunlight on its drug disclosure regulations.
This seems like a perfect opportunity for Democrats and Republicans to find common ground and pass legislation requiring country-of-origin labeling for medicines to include global sourcing of active ingredients.
The production of acetaminophen and other generics may be finalized in this country, but if the components all come from China or India, that’s a data point consumers are entitled to know.
And if it’s too much hassle to put that on the label, at the very least such transparency should be introduced online for anyone interested in knowing more about what they’re putting in their mouth.
As it stands, our disclosure rules appear designed more to hide countries of origin rather than reveal them.
At times like these, that’s not very helpful.
More to Read
Inside the business of entertainment
The Wide Shot brings you news, analysis and insights on everything from streaming wars to production — and what it all means for the future.
You may occasionally receive promotional content from the Los Angeles Times.