2 Research Groups Test Promising Vaccine for Malaria
- Share via
When Alphonse Laveran identified the parasite that causes malaria more than 100 years ago, few at the time realized that the French army physician had merely set the stage for a century of research that has left countless scientists baffled and frustrated.
Repeatedly, researchers seeking a vaccine against malaria have been outwitted by the parasite. Until now.
Almost without notice, two groups of U.S. scientists have come up with a promising test vaccine against malaria that, if proven safe and effective, would also be the first vaccine against a human parasite--a major achievement in itself.
Such a vaccine could help reduce the enormous toll taken by malaria, which each year strikes about 300 million people, killing about 5 million of them, mostly in the Third World.
In much of the developed world, malaria has largely been eliminated as a result of public health measures and insect control programs. But the disease is still rampant in the Third World because mosquitoes continue to develop resistance to pesticides and anti-malaria drugs eventually lose their effectiveness against the parasite.
But if malaria is ever to be conquered, more than just newer and more effective drugs and better insect-control programs are needed. A vaccine may be crucial, many researchers believe.
And today a vaccine is quietly being tested on a small group of people at Walter Reed Army Research Institute in Washington, where researchers have spent the past 30 months developing the preventive.
“It seems to be a safe, benign vaccine,” said Dr. Wayne Hochmeyer, chief of immunology at the research institute and a developer of the vaccine with Dr. Louis Miller of the National Institute of Allergy and Infectious Diseases.
A second vaccine, developed by Drs. Victor and Ruth Nussensweig of New York University, is expected to undergo early human trials this summer.
Such a vaccine could also lead to new insights into ways to contain a host of other parasitic killers and cripplers in tropical Third World countries.
Propelled by the same technological advances--especially in genetic engineering--that have made a test vaccine against malaria feasible, scientific interest is also mounting in other tropical diseases that disrupt the lives of hundreds of millions of people as well as the economies of the struggling nations in which they live.
“By committing new resources and technology toward finding solutions for medical problems largely ignored by Western medicine, we have the potential for bringing new aid to a large portion of the world’s people,” said Dr. Gary Schoolnik, chief of Stanford University’s “geographic medicine” branch.
But another Stanford researcher, Dr. Paul Basch, cautioned against too great a reliance on biotechnology alone to solve the health problems of the Third World through vaccines and drugs.
“Improving the living conditions of people is the long-term answer,” he said, noting that Americans suffered from many of the illnesses now running rampant in developing countries--including malaria, cholera and diarrhea--until a steadily improving economy made possible better sanitation and insect control.
Among the target diseases are schistosomiasis, a chronic, debilitating condition caused by a parasitic worm carried by snails that infects 125 million people in Africa and Southeast Asia; African sleeping sickness, a fly-borne illness that threatens 45 million people on that continent; and leishmaniasis, another insect-borne disease that infects many body organs and is a major public health problem in Latin America and many other parts of the world.
In addition, there is dengue fever, which struck many GIs during the Korean War, and is still common there and in parts of Central America and the Caribbean; Chagas disease, a South American viral disease that can damage the heart; and leprosy, river blindness and a dozen other lesser known but equally devastating afflictions.
No vaccine exists for any of those diseases. And for some--such as the feared African sleeping sickness--none is likely because of the organism’s uncanny ability to continually change its outer coating, thus presenting an ever-changing target for the body’s immune system, researchers say.
Although some progress to prevent sleeping sickness was being made during the 1960s and ‘70s in some East African countries through control of the tsetse fly, epidemics have recently occurred in Uganda, according to the World Health Organization.
Even AIDS, or acquired immune deficiency syndrome, has not generated nearly the fear that was evoked by sleeping sickness around the turn of the century in equatorial Africa, according to Dr. John Boothroyd, a Stanford molecular biologist. One epidemic alone had wiped out two-thirds of the people living around Lake Victoria, he said.
England and Belgium, two Central African colonial powers at the time, were so concerned that they established major centers to come up with a cure. It was that quest, according to Boothroyd, that spawned the science of chemotherapy.
But 80 years later, drug treatments for many tropical diseases still leave much to be desired.
Still, over the years, a handful of pharmaceutical companies and university researchers have persevered, and now, in a few instances, are making some headway using newly developed drugs. Schistosomiasis and river blindness, an affliction that occurs in parts of West Africa, are two examples.
One reason for the increasing interest in this country in diseases that rarely, if ever, occur here is the growing number of Americans who visit or work in tropical countries, experts say.
The Office of Technology Assessment, an agency that advises Congress, estimates that about 5 million Americans each year are at risk of developing a tropical disease. Besides tourists, they are largely the military and employees of multinational companies working in the Third World.
It is the military and long-term residents in malaria areas who probably would be among the first beneficiaries when a malaria vaccine becomes available.
But Hochmeyer said it will be another year before enough data can be collected to know whether the vaccine being tested affords enough protection to make it worthwhile to begin larger-scale clinical trials in various parts of the world where the disease is endemic. Those trials could be several years away.
In the experiment now under way, only 15 people are involved, including several of the researchers who developed the test vaccine.
Researchers reached this point only after they achieved a better understanding of the parasite’s complex outer coating--a necessary prerequisite to the design of a vaccine. The development of a vaccine also had been stymied by the inability to culture the parasite--an obstacle that has been overcome through application of genetic engineering.
But even if the human trials prove successful, the complex life cycle of the malaria parasite may limit the vaccine’s usefulness because the parasite passes through three stages inside the human. The vaccine would be effective against only the earliest of those stages.
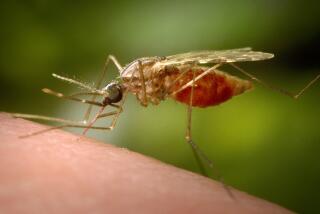
The life cycle begins with the bite of a female Anopheles mosquito that is carrying malaria parasites in her salivary gland. As she sucks out blood, the parasite--at that stage of its life cycle known as a sporozoite--enters the human’s blood stream and circulates throughout the body.
Within about an hour, however, the sporozoites leave the bloodstream and invade the liver, where after a week or two they transform into a second stage called the merozoite. Rupturing the liver cells in which they developed, the merozoites then return to the bloodstream and invade red blood cells.
Some of them undergo a third transformation into a state known as the gametocyte. A mosquito that bites the individual at this point is apt to suck up the gametocytes, which, nestled inside the insect, develop into sporozoites. When the sporozoites migrate to the mosquito’s salivary gland, they are injected into the next human that the mosquito bites, beginning the cycle again.
Both the New York University and the Army vaccine are intended to interrupt the cycle only at the sporozoite stage. If the vaccine accomplishes its purpose, all of the sporozoites that have been injected by the mosquito will be neutralized.
But because sporozoites remain in the bloodstream for only an hour or two before invading the liver, the antibodies that are produced by the vaccine must act swiftly.
Otherwise, the escape of even a single sporozoite into the liver may allow the life cycle to continue and the disease to occur despite the vaccine. This is because the outside coatings of the stages that develop in the liver and in the red blood cells differ from the sporozoite’s coating, rendering the antibodies produced by the vaccine ineffective against the parasite in those two stages of development.
Scientists believe that to protect individuals who live permanently in malarious areas, additional vaccines made of antigens obtained from the second and third stages of the parasite’s life cycle will have to be incorporated in the new vaccine. That could be many years away, although researchers from a number of laboratories throughout the world already are working toward such vaccines.
Still another obstacle to the eventual control of malaria is the fact that the disease in man is caused by four different species of the parasite.
Both the Army and the New York University vaccines are intended to protect against only one species, called Plasmodium falciparum , the most lethal of the four. But the complete control of malaria--especially for people who live in malaria areas, in contrast with those who visit temporarily--may require a separate vaccine for each species, Hochmeyer said.
The U.S. Agency for International Development, a federal agency that has funded much of the malaria research in this country, will continue to fund it until a vaccine exists for all three stages and all four species, according to Dr. James Erickson, manager of the vaccine program for the past 10 years.
Erickson said the agency, which has spent $20 million on a malaria vaccine, began the project 21 years ago midst the scoffing of many researchers of the day who believed that such a goal was impossible.
“We did it because nobody else would do it,” Erickson said. “Now everybody is getting on the bandwagon.”