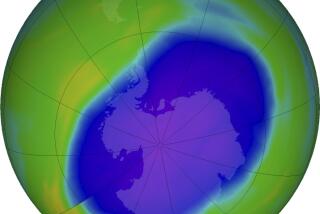
Ozone Layer ‘Hole’ Laid to Activity of Sunspots
- Share via
The “hole” in the earth’s protective ozone layer over Antarctica may have been caused by increased sunspot activity rather than by man-made chemicals, a National Aeronautics and Space Administration scientist reported today.
The increased solar activity produces its most dramatic effect over Antarctica because of its long, lightless winter and could explain the smaller decreases in ozone concentrations that have appeared at other sites around the world, according to Linwood B. Callis of NASA’s Langley Research Center in Hampton, Va.
Significantly, he said in a telephone interview, the solar activity reached its peak at the start of 1980 and the ozone layer should now be recovering from its effects. The beginning of this recovery can be seen in the most recent satellite data, which has not yet been published, he said.
Other scientists, however, are not completely ready to accept Callis’ theory. A team of scientists now in Antarctica to study the ozone hole reported in a press conference two weeks ago that they could find no evidence of the increased levels of nitrogen dioxide that Callis says were produced by the solar activity.
Significant Role
The scientists said they believe that the man-made chemicals--called chlorofluorocarbons--play a significant role in causing the hole in the ozone layer.
The chlorofluorocarbon industry, itself, has apparently come to accept the once-controversial theory that the chemicals are destroying the ozone layer. Earlier this month, the industry’s trade association conceded that a worldwide ceiling should be placed on production of the chemicals. The largest manufacturer, the DuPont Co., has even suggested that total production may have to be reduced to prevent an ecological catastrophe.
If Callis’ theory is correct, then, the ozone problem may not be as severe as many scientists now believe.
Ozone is a highly reactive, naturally occurring ingredient of the stratosphere--the segment of the atmosphere extending from 9 to 30 miles above the earth’s surface--that is produced from oxygen by sunlight. It screens out about 99% of the invisible ultraviolet radiation in sunlight.
High intensities of ultraviolet radiation are harmful to life, and scientists agree that a significant depletion of the ozone layer and the accompanying increase in the amount of ultraviolet light reaching the earth’s surface could cause a large increase in skin cancer, harm many species of animals, plants and fish, and perhaps even adversely affect climate.
Chlorofluorocarbons are used in refrigeration systems and for the production of plastic containers for fast foods. Their use in aerosol cans has been banned in the United States since 1979 but they are still utilized for that purpose in many foreign countries. These chemicals are not destroyed in the environment and rise to the stratosphere. There, sunlight breaks them down into smaller fragments that destroy ozone.
Scientists have been measuring the amount of ozone in the stratosphere since the chlorofluorocarbon theory was first advanced in 1974, and recent evidence suggests that the total amount of ozone in the stratosphere has fallen by about 3% since then, with decreases of as much as 14% in the mid-latitudes of the southern hemisphere.
Last year, however, scientists found that a large hole, about the size of the United States, develops in the ozone layer over Antarctica during the southern hemisphere’s spring. Ozone levels in the hole were as much as 45% lower than levels that had been measured during the 1960s.
More recently, researchers have found a similar, albeit smaller and less pronounced, hole that develops in the arctic in the northern hemisphere’s spring.
Most scientists have assumed that the formation of these holes was related to the increasing use of chlorofluorocarbons, but Callis and Murali Natarajan of SASC Technologies Inc. in Hampton543257191journal Nature that the holes more likely have a natural cause.
75% Above Normal
Callis and Natarajan studied four sets of satellite data and found that the concentration of nitrogen dioxide and related chemicals in the thermosphere--the segment of the atmosphere above the stratosphere--was as much as 75% above normal during the period 1979 to 1984. This interval coincided with an increase in sunspot activity that peaked in the winter of 1979-80.
“It’s well-known that you get an increase of (these compounds) during periods of sunspot activity,” Callis said. Sunspots, which occur in cycles that peak every 11 years, increase the intensity of radiation and magnetic fields that reach the earth.
Callis speculated that during the long, lightless southern hemisphere winter, nitrogen compounds from the thermosphere are transferred to the stratosphere. When light returns in the spring, it touches off a period of intense chemical activity that causes the ozone loss. Normal air flow then causes the air in the hole to mix with the rest of the atmosphere, so that the hole disappears.
A key point, he said, is that “it takes three or four years for the chemicals to work themselves out of the stratosphere.” The periods of maximum ozone depletion should thus lag behind the sunspot cycle, and should have reached a maximum in 1984-85, declining thereafter. Callis believes that he has seen the beginning of that decline.
A similar phenomenon should occur in the northern hemisphere, he said, but the effect is smaller because there is a much greater mixing of the atmosphere in that hemisphere. The increased mixing would explain why the ozone depletion is smaller over the arctic.