First AIDS Home Test Kits Gain Approval From FDA
- Share via
WASHINGTON — The Food and Drug Administration approved the first home AIDS test kit Tuesday, acknowledging that conditions surrounding the controversial idea have changed substantially in recent years.
“We are confident that this new home system can provide accurate results while assuring patient anonymity and appropriate counseling,” FDA Commissioner David A. Kessler said in a statement.
The agency said that it was influenced by technological advances in the accuracy of the tests, the availability of more effective therapies for people who are infected but not yet symptomatic, and the public health benefits of having more people know whether they are infected.
“Science and technology have evolved to the point where we believe the benefits of this new product outweigh the risks,” Kessler said.
The federal Centers for Disease Control and Prevention have estimated that more than 60% of Americans who engage in high-risk AIDS behaviors have not been tested. Until now, all HIV testing was conducted under the supervision of a health professional, either in a clinic or a physician’s office.
“Too many Americans do not know their HIV status,” said Health and Human Services Secretary Donna Shalala. “Knowledge is power, and power leads to prevention.”
*
The concept--initially rejected by the FDA six years ago--has been hotly debated.
Advocates of the home kits have argued that their availability would encourage testing by more people, thus prolonging or saving lives. Many individuals are reluctant to seek testing from clinics or private physicians, particularly in small towns or rural areas.
Opponents have countered that the kits hold the potential for abuse--such as pressure from spouses, employers or insurers--to submit to testing. They are worried that the test might also be used by teenagers or children who might not understand the implications of test results.
Furthermore, they are concerned about the impersonal nature of the process, which does not involve face-to-face counseling.
The product, known as Confide, was developed by Direct Access Diagnostics, a Johnson & Johnson company, in conjunction with Chiron Corp. Initially the kits will be available over the counter only in Texas and through the mail via a toll-free number in Texas and Florida. They will be available nationwide in early 1997.
Jeffrey Leebaw, a spokesman for Johnson & Johnson, said that the company “wants to move slowly” in introducing the kits because “it’s a new product, a serious product, and the company wants experience with it before going into a national launch.”
Texas and Florida were selected because “they are manageable size populations, are culturally diverse and have a significant amount of AIDS and HIV,” he said.
The kits will cost about $40 retail, he said.
Mary Fisher, the prominent Republican who heads the Family AIDS Network and who is HIV-infected, was among those who in 1994 urged an FDA advisory committee to recommend approval of the kits. She applauded the decision Tuesday.
“This gives privacy and anonymity for those who need it and who feel this is the only way they would get tested,” she said. “We are all at risk, and this gives us one more option for prevention.”
But Aimee Berenson, AIDS Action Counsel’s legislative counsel, acknowledged the potential benefits of the kits, but she also warned: “People should not forget all the potential risks. This is not the answer to stopping the spread of HIV. The answer is to make sure that we’re educating people so they know they’re at risk.”
*
Dr. Gary Noble, vice president of medical affairs for Direct Access Diagnostics, said that the impetus for testing gained momentum with the development of treatments that “can increase the quality and length of life” for many infected individuals. Moreover, a recent treatment breakthrough reduces the transmission from infected mothers to babies.
Noble was referring to research which has shown that use of the antiviral AIDS drug AZT can sharply reduce transmission of the virus from mother to fetus.
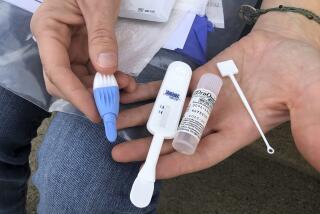
The kits contain pretest counseling booklets about HIV and AIDS. Using a lancet enclosed with the materials, the person takes a finger-stick blood sample and places it on a designated area of a test card that has been coded with a unique identification number.
The card is then mailed in a protective envelope to a certified laboratory for HIV-1 antibody testing. The lab work is identical to that conducted in other settings: Samples that test positive are retested and then confirmed with another more specific test.
*
Seven days later, results can be obtained by calling a toll-free number and asking for results for the identification number supplied in the kit.
If the result is positive, professional, certified counselors, who speak both Spanish and English, will be connected to callers directly. They will offer local medical referrals, if needed, and encourage the caller to seek medical care. The conversations will be confidential and anonymous.
Negative results will be provided by an automated message, although everyone will be given the opportunity to speak to a counselor.
Also, all negative test results include an explanation of the so-called window of time between infection and the development of antibodies, usually about a month. During that time, individuals will test negative even though they are infected.
(BEGIN TEXT OF INFOBOX / INFOGRAPHIC)
How the Test Works
The new testing system is composed of three integrated components: an over-the-counter home blood collection kit, HIV-1 antibody testing at a certified lab and a center that provides test results, counseling and referral anonymously. The kits will cost $40 retail.
Step 1: Blood sample taken at home
Step 2: Sample placed on test card precoded with a unique identification number to maintain anonymity.
Step 3: Test card is mailed in a protective envelope to a certified laboratory for testing. Positive samples are tested twice.
Step 4: Results available in seven days by calling a toll-free number.
Sources: Times wiere reports; Johnson & Johnson
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.