Just 1 Little Neutron Can Make a World of Difference
- Share via
Little things mean a lot--and not only when it comes to romance. The natural world is replete with small differences that have enormous effects.
A difference in temperature of one degree causes a road to freeze over in the rain, sending cars into skids. The difference of one electron changes a sedate, unreactive gas (neon) to a highly reactive metal (sodium).
In the animate world, an almost imperceptible change in intonation tells you someone is angry. The genetic difference between humans and African great apes is less than 1%. Small differences in animal traits (a stronger beak, a longer neck) evolve over time into entirely different species.
Indeed, according to writer Annie Dillard, the difference between plants and animals is not as great as you might suspect: The lifeblood of green plants is the molecule chlorophyll, made up of 136 atoms of hydrogen, carbon, oxygen and nitrogen arranged in a ring around a single atom of magnesium; the lifeblood of people is hemoglobin (blood), made up of 136 atoms of hydrogen, carbon, oxygen and nitrogen arranged in a ring around a single atom of iron.
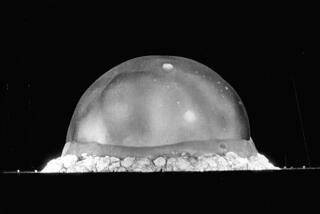
The big effects of small differences came into clear view last year during the controversy over the launch of NASA’s Cassini spacecraft to the ringed planet Saturn. To keep its electronics warm and running in the cold outer reaches of space, Cassini carried 72 pounds of radioactive plutonium. Plutonium is scary stuff: It fuels nuclear bombs. Protesters insisted that 5 billion people could suffer radiation effects if Cassini crashed into Earth during its planned fly-by in 1999.
The debate over Cassini’s safety was confusing in part because the kind of plutonium carried on Cassini isn’t any good for making bombs, but it is 250 times more radioactive than bomb-grade plutonium. The same element--but with a very different character.
What’s the difference? One lousy little neutron in the atom’s nucleus. Plutonium 239--used for bombs--splits easily, setting off chain reactions. Plutonium 238--used to power spacecraft--does not split easily, but spits out radiation at an enormous rate; if it lodges in someone’s lungs, it can be a death sentence.
The mystery is: Neutrons are unimaginably small. They have no electric charge. How can they make such a big difference?
According to Caltech nuclear physicist Steven Koonin, a plutonium nucleus is a lot like a drop of water. Normally, the surface tension of water molecules around the edge of the drop creates a kind of skin that keeps the droplet intact. But opposing forces--say, the friction of a car window tearing the drop in two--can break the skin and cause the drop to fall apart.
“That’s a pretty good description for what goes on in the nucleus [of plutonium],” said Koonin. That one extra neutron can change the shape of the nucleus from a spherical drop into a dumbbell shape. And dumbbells are much easier to split than spheres.
The extra neutron, in other words, can be the tipping point.
Physicists aren’t surprised when small differences make big differences. After all, they’re here in the first place because of an extremely small difference that no one quite understands.
When matter first came into being after the birth of the universe in the Big Bang, it was created in two opposite varieties: matter and antimatter. When matter and antimatter meet, they annihilate each other in a burst of pure energy.
*
So if the universe began with equal parts of both, why are we here? Why is anything here?
The answer is that there must have been an infinitesimally small imbalance in the creation equation. Matter must have, so very slightly, outweighed antimatter. So when the matter and antimatter particles annihilated, a small excess remained.
That excess is us.
Nobody knows quite how the imbalance popped up in the first place.
Just as startling, cosmologists have calculated that a universe with a slightly greater amount of matter and energy would have collapsed on itself almost as soon as it formed. A universe with slightly less energy would have expanded into a diffuse cloud, with no atoms, stars or planets.
In the end, the romantics are right. There’s a good reason to make much ado about very little.