Vaccine May Be the Key in Cervical Cancer Fight
- Share via
U.S. researchers have successfully tested a vaccine against human papilloma virus, a feat many consider the first step toward the eventual prevention of most cases of cervical cancer, which is caused by the virus.
Widespread use of the vaccine, which could occur in as little as five years, could eventually result in an 85% reduction in the worldwide number of 450,000 new cervical cancer cases and 250,000 deaths each year.
Such an effective vaccine “would have profound effects,” said Dr. Christopher P. Crum of Brigham and Women’s Hospital in Boston, who added that papilloma infections could eventually fade away, as have polio, smallpox and diphtheria.
Another team reports today that a vaccine against herpes simplex virus 2, the primary cause of genital herpes, is 74% effective in women who have never been exposed to a herpes virus. Genital herpes afflicts one in five Americans over age 12.
“This is the first clinically relevant success we have had in the entire field,” said Charles Ebel of the American Social Health Assn., which has been leading anti-herpes campaigns. “Nothing has worked at all until now.”
Some data suggest that herpes also contributes to cervical cancer, so the development of a vaccine against it would provide a double whammy.
“This is the first time that this type of therapeutic intervention is available for a cancer that affects hundreds of thousands of women worldwide,” said Dr. Beth Carlin of Cedars-Sinai Medical Center in Los Angeles.
Both vaccines are now entering larger clinical trials. The herpes vaccine also could be available for clinical use in as little as five years.
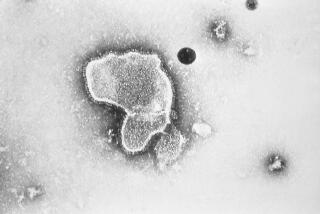
Since human papilloma viruses were discovered two decades ago, researchers have identified 20, five of which are involved in triggering cervical cancer. But one, HPV-16, is by far the most common, infecting an estimated 20% of the U.S. population and causing at least half of the cancer cases. The new vaccine specifically targets HPV-16.
The vaccine is made of virus-like particles, grown in yeast, that have all of the surface proteins of the virus but none of its genetic information. It is made by Merck Research Laboratories and administered in three injections over six months. Merck paid for the latest study.
In the study, a team led by Laura A. Koutsky, an epidemiologist at the University of Washington, tested 1,533 women ages 16 to 23. Half received the vaccine and half a placebo. The women were studied for an average of two years, and they will be followed for an additional two.
The team reports in today’s issue of the New England Journal of Medicine that 41 women in the placebo group developed HPV-16 infections during the course of the study, but that none of the women receiving the vaccine did. Nine cases of HPV-16-related cervical intraepithelial neoplasia -- a precursor of cervical cancer -- occurred among women who received the placebo, but none among those who received the vaccine.
The group is now starting trials of a vaccine that targets not only HPV-16, but also three other papilloma viruses. The four viruses account for 70% of cervical cancers and 90% of genital warts.
“The implications of having a vaccine are enormous,” Koutsky said. Screening for cervical cancers with Pap smears has reduced the yearly number of deaths in the United States to about 4,100, but the tests are too expensive for most developing countries. “A vaccine could make a real impact in those countries,” Koutsky said.
It could have a different kind of effect in this country, Carlin said. Many women with cervical cancer must undergo hysterectomies as treatment, leaving them infertile. Preventing infections would eliminate most of those cases, she said.
A majority of positive Pap smears, furthermore, are associated with an HPV infection rather than cervical cancer, and each positive test requires expensive and time-consuming follow-up tests to rule out disease, Crum said. A decrease in infections would eliminate as many as 70% of the false-positives, alleviating the stress on the women involved and potentially saving the health-care system billions of dollars every year.
At least two other HPV vaccines are now in clinical trials, and researchers are looking at innovative ways to produce others. Researchers at the University of Rochester in New York, for example, are developing potatoes, tomatoes and bananas that contain the HPV virus-like particles. Tests in animals have shown that these particles produce immunity similar to that produced by conventional vaccines. But they could have a major advantage in that they would be cheaper to produce and less resistant to degradation in tropical countries.
The herpes vaccine, produced by GlaxoSmithKline Biologicals, is targeted at herpes simplex virus-2 (HSV-2). A related virus, HSV-1, primarily causes oral herpes, but it can produce the genital form as well.
Herpes viruses can cause painful itching and sores, but most people who are infected show no symptoms, which is why it is spread so easily. Recent evidence suggests that a co-infection of HPV and HSV-2 doubles the risk of cervical cancer. Herpes infections are also thought to accelerate the spread of the human immunodeficiency virus. And babies who contract the herpes virus from their mothers are at a high risk of death. From 2,500 to 3,000 such cases occur in the United States each year.
A team led by Dr. Lawrence Stanberry of the University of Texas Medical Branch in Galveston enrolled 978 women and 1,736 men whose partners had genital herpes. Half received the vaccine in three injections and half a placebo.
The team reported in the New England Journal of Medicine that the vaccine protected 74% of women who had never been exposed to either HSV-2 or HSV-1. It was much less effective in women who had been exposed to HSV-1, and for reasons that mystify the researchers, was not effective at all in men.
Researchers expect to make improvements in the efficacy of the vaccine. But even if they do not, Ebel said, mathematical modeling suggests that widespread use of a vaccine that is 74% effective only in women could still produce a significant reduction of cases in both men and women.
The National Institute of Allergy and Infectious Diseases said Wednesday that it will begin enrolling 7,550 women this week in a much larger trial. Enrollment will be restricted to women who have not been exposed to either form of the virus.
Because neither vaccine is effective on a person who has been exposed to the virus, researchers say it may necessary to vaccinate girls before they have any sexual experiences, probably around age 12 or 13. “It would eventually become part of our vaccine protocol,” Carlin said.