Stubborn Killer Is Deciphered as Scientists Map Malaria DNA
- Share via
NEW YORK — In a major advance against a disease that kills 3 million people every year, researchers have deciphered the complete genetic makeup of the malaria parasite and the mosquito that transmits it, an international coalition announced Wednesday.
By unraveling all the genes that go into this lethal partnership of pest and pestilence, scientists hope to find better ways to treat a disease that has resisted every attempt by modern medicine to eliminate it.
“This truly is a landmark accomplishment,” said Dr. Anthony S. Fauci, director of the National Institute of Allergy and Infectious Diseases, which helped fund the research. “It is the beginning of a new era of science in the study of malaria.”
Despite decades of systematic public health efforts, there are half a billion new cases of malaria every year, with more cases of the illness in Africa today than at any time in history, researchers said. Almost 8,000 people die every day of malaria, most of them infants and children. For the first time in 20 years, malaria recently turned up in mosquitoes in the United States.
Throughout the world, the mosquitoes that carry malaria have become resistant to most insecticides used to control them, while the disease itself has become resistant to the drugs most commonly used in treatment. In a real sense, malaria today is the product of efforts to control it.
The findings announced Wednesday make for an unprecedented moment in public health: For the first time, researchers have in hand the complete genetic sequence of a disease parasite, the insect that transmits it and the human host who is racked by its killing fevers.
The resulting genetic insights into malaria could lead to new insecticides, effective vaccines, better medicines and genetically engineered insects that break the cycle of disease transmission.
By looking for biochemical vulnerabilities in the malaria parasite itself, researchers already have identified five potential targets for new medications.
Yet so many other obstacles remain--technical, environmental and financial--that it may be a decade or more before the unraveling of the genomes has any direct effect on the disease, several malaria control experts said.
They tempered their enthusiasm for the news with caution about the difficulties of translating such research breakthroughs into effective treatments that people in the world’s most underdeveloped countries--where 90% of the illness is concentrated--can afford.
“Genome sequences alone provide little relief to those suffering from malaria,” said molecular geneticist Russell Doolittle at UC San Diego. “Translating all of this information into new treatments and cures is not a trivial exercise.”
The genome breakthroughs, announced in Washington and London, arise from the collaboration of 160 researchers in 10 countries. One international team sequenced the mosquito genes, while the other sequenced the genes of the malaria parasite. In all, the effort took six years.
Research papers concerning the malaria genome are being published today in the journal Nature. Technical papers on the mosquito genome were released Wednesday on the Internet by the journal Science.
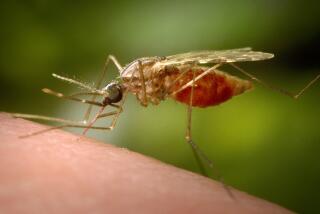
A consortium of 19 laboratories led by Robert Holt, a senior scientist at Celera Genomics Inc. in Rockville, Md., deciphered the genetic sequence of the most deadly malaria mosquito--known formally as Anopheles gambiae--a living hypodermic needle with an instinctive thirst for human blood.
From a public health standpoint, the researchers said, it is “the most important insect in the world.” In all, 60 species of mosquito transmit malaria. The malaria mosquito breeds in places such as water barrels, irrigation ditches, tires and the tiny pools that gather in footprints in the mud after it rains. Dozens of species can swarm in the same area.
Primarily responsible for transmitting malaria in Africa, it is the biochemical product of a genome containing approximately 278 million base pairs of DNA that encompass about 14,000 genes, the Celera team determined.
By comparison, the human genome has about 3 billion base pairs of DNA and contains an estimated 35,000 genes.
An international team led by researcher Malcolm Gardner at the Institute for Genomic Research in Rockville unraveled the genetic chemistry of the deadliest and most common of the four variants of the parasite that causes the disease.
The malaria parasite, known formally as Plasmodium falciparum, owes its existence to a genetic sequence containing 24 million base pairs of DNA, with about 5,300 genes.
Its genome was smaller, more complex and harder to piece together than scientists expected.
At least half of the malaria genes appear to be unique, appearing in no other gene database so far. And almost 80% of the parasite’s genome is a stuttering gibberish composed of just two of the four letters in the chemical alphabet of DNA, making it very difficult for researchers to tell where pieces of the sequence start and stop.
“This was perhaps the most difficult genome we ever have sequenced, despite the fact it is smaller than many,” Gardner said. “It was a huge jigsaw puzzle, with many pieces that did not quite fit together.”
The complete genetic script for the parasite and the pest that carries it from person to person may help unlock the complicated three-stage life cycle of malaria.
The new genetic information gives scientists the opportunity to understand a biochemical parry and thrust between species that have been evolving for thousands of years, said tropical disease expert Dyann Wirth, head of Harvard University’s Malaria Initiative. That may reveal how the malaria parasite is so adept at evading the immune system defenses of those it infects.
“This is a huge advance,” Wirth said. “We can look now for the Achilles’ heel.”
The mosquito passes the malaria parasite to humans when the infected insect feeds on their blood. As it sucks up blood, the insect injects saliva that contains the parasite.
Once in the bloodstream, the parasite migrates to the liver, where it multiplies, forming dense cysts. The cysts eventually burst, flooding the bloodstream with parasites, setting in motion the next round of malaria’s infectious life cycle.
When the next mosquito bites, it sucks up the parasite. The parasite reproduces and its young migrate to the mosquito salivary glands, where they are injected next time the insect bites someone.
Without the mosquito, the parasite languishes and dies. Without its blood meal, the biting mosquito cannot breed. It needs the blood to nurture its eggs.
The Anopheles gambiae mosquito is so deadly, in part, because it feeds almost exclusively on human blood, unlike other closely related mosquito species. Scientists generally agree that the malaria mosquitoes somehow can sniff out their preferred human prey, apparently attuned to the rank smell of feet, among other things.
In all, researchers have identified 276 genes involved in the mosquito’s sensory systems. At least 19 of the newly sequenced genes appear to be involved in the insect’s ability to detect odors, including the smell of human blood. That may offer a way for researches to derail the insect’s ability to find and feed on human beings.
“If we can identify the receptors that mosquitoes use to smell humans, we should be able to design novel repellents that can substantially reduce the incidence of malaria, West Nile encephalitis, dengue and yellow fever and other mosquito-borne diseases,” said biologist Laurence Zweibel at Vanderbilt University, who helped decipher the genes.
Malaria experts are even more anxious to identify the genes responsible for the insect’s growing resistance to insecticides used to control it.
Other research teams are seeking ways to make the insect itself resistant to the disease parasite.
Earlier this year, researchers were able to genetically modify mosquitoes so that some forms of the parasite could not survive inside them. Scientists now have identified a few genes in mosquitoes that reduce the natural transmission of the most lethal malaria parasite.
“The new genes we have found are the first ones that make the Anopheles mosquito highly resistant to real, natural populations of the most deadly of the human malaria parasites, as opposed to laboratory parasite strains,” said New York University parasitologist Kenneth Vernick, who led the research team. As many as half the mosquitoes in the wild may carry genes for parasite resistance.
But the new genome information reveals that the parasite appears unusually well-equipped to evade such molecular defenses, Wirth said.
The parasite’s ability to mutate also allows it to quickly develop resistance to drugs.
“I was surprised,” she said, “at the number and variety of genes that are apparently involved in the evasion of the immune response.”