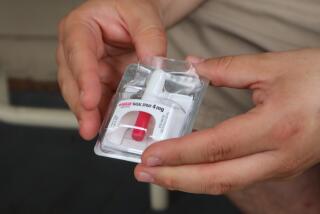
Access sought for morning-after pill
- Share via
The morning-after pill is safe and effective enough that it should be sold over the counter, a manufacturer says, and it is asking the federal government for permission to do so.
Women’s Capital Corp., maker of the Plan B emergency contraceptive, will submit an application to the Food and Drug Administration today asking for approval to sell the product without a prescription. The request is expected to generate protests from antiabortion groups that say the pill causes abortion.
Plan B was approved as a prescription drug in 1999; it is one of two such products currently available. California is one of a few states that allow emergency contraceptives to be sold without a prescription. They can only be bought, however, from certain pharmacists who work with doctors to decide when the pills are appropriate.
Sharon Camp, president of Women’s Capital Corp., says over-the-counter status would make the drug more accessible. Emergency contraception consists of a small dose of birth control pills that, if taken within 72 hours of intercourse, can help reduce the chance of pregnancy by 89%. It is thought to work by delaying ovulation, preventing fertilization or inhibiting implantation of a fertilized egg. The main side effect is nausea.
“This is an idea whose time has come,” Camp said. “There are a large number of medical organizations and women’s health groups who believe we need to remove the prescription requirement because women often cannot get a prescription written and get it filled within the 72 hours needed to use it.”
Although emergency contraception is different from the abortion pill mifepristone, some antiabortion groups oppose its use. This year, the Senate rejected a proposal for a national campaign to raise awareness about the morning-after pill.
“It works as an abortifacient, meaning it can take the life of a pre-born child,” said Judie Brown, president of the American Life League, an antiabortion group in Virginia. “It poses risks to the woman taking it. There have been no studies whatsoever on the long-term effects of this pill regimen, especially the effect on teenagers.”
The FDA is expected to make a decision in 10 months.
James Trussell, director of the Office of Population Research at Princeton University, said there are scores of studies on the pill’s safety and predicts the FDA will approve the over-the-counter application. But Trussell said he doubts over-the-counter status would greatly increase use of the product.
“It will be helpful to women who know about it,” he said. “But [access] is not the main problem. The main problem in the United States is that many women don’t know about emergency contraceptives.”
Plan B is expected to cost $20 to $30, Camp said. Many health plans pay for Plan B prescriptions, but most women still have the costs of a doctor’s office visit and prescription co-payment.