Easing access to cardiac shockers
- Share via
WASHINGTON — Some have called it a jump-starter for the human heart. But the maker of a new automatic external defibrillator wants Americans to think of it as just another safety device for the home -- vital and user-friendly, like a fire extinguisher.
Whether the HeartStart Home device will become standard issue for the safety-conscious home depends largely on whether the Food and Drug Administration, in a decision expected in the next several weeks, will allow the devices to be sold over the counter -- that is, without requiring a doctor’s prescription.
If approved for sale to the broader public, it remains to be seen whether health-conscious Americans will ante up almost $2,000 to put a rescue device for victims of cardiac arrest on a shelf at home.
Dr. Bram Zuckerman, director of the FDA’s division of cardiovascular devices, signaled likely approval, after an advisory panel meeting last week, for the device to be sold without a prescription. Zuckerman said the panel’s consensus was that the device, “when used by the intended lay user is a safe device” and that its effectiveness had been demonstrated.
The FDA deliberation comes as heart defibrillators, long operated only by doctors and trained medical staff, have become a widespread presence in public buildings, shopping malls, health clubs and schools. If cleared for sale by the FDA, the heart-shockers could turn thousands of untrained Americans into emergency rescuers.
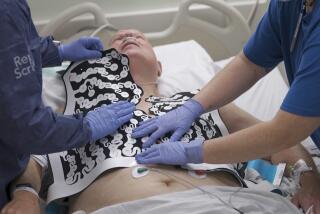
The HeartStart Home Defibrillator, made by Philips Medical Systems, is the first to have been designed for use by those without specific training. Philips scientist Carl Morgan, who testified last Thursday before the FDA panel, called the HeartStart Home device a “once-in-a-lifetime” aid that can be operated by a novice and save a life in the crucial first minutes of a heart attack. Roughly the size of a lunchbox, it holds two sets of pads: one to deliver a shock to an adult heart, the other to revive a child.
More than 300,000 Americans suffer sudden heart attacks each year -- more than two-thirds of them, by most accounts, in their homes. For those victims, the first five to 10 minutes after the onset of major symptoms is critical to heading off irreversible heart failure. Experts believe that every minute that passes before normal heart rhythm is restored cuts the probability of survival by roughly 10%.
Morgan said some safety precautions had been built into HeartStart’s design: The device won’t deliver a jolt of electricity if a person’s heart is beating normally, and it won’t work on someone whose heart has stopped beating altogether.
At last week’s meeting, the FDA panel debated whether putting more defibrillators into use -- and into untrained hands, specifically -- would endanger some lives, even while it saves more. Although use of heart defibrillators has risen steadily since 1996, so have malfunctions, according to a report presented to the panel. Reported malfunctions have more than tripled in the last eight years, with “associated” deaths rising even faster: from 20 in 1996 to more than 100 yearly in 2001, 2002 and 2003.
From 1996-2003, manufacturers of the devices reported 7,044 malfunctions involving all defibrillators distributed in the United States. FDA advisors acknowledged that spotty information about the cause and nature of the malfunctions made the significance of the new data difficult to discern.
Defibrillators work by delivering a significant electrical shock to the malfunctioning heart -- a jolt that, in many cases, restores the heart to its normal rhythm. Because automatic external defibrillators have been riding with police and paramedics in the early 1990s, and began being placed in public venues in the late 1990s, survival rates for victims of sudden cardiac arrest have moved steadily upward.
But a study published in the journal Resuscitation in August 2003 found that the survival rate for victims of sudden cardiac arrest at home were 30% to 50% lower than those whose heart attack occurred in a public place.
Kelli Harris is one of the lucky ones. In December 2002, the athletic, 29-year-old sports club employee’s heart went wildly out of rhythm during a workout in Portland, Ore. As Harris was doing squats with a trainer one evening, she fell backward with a howl.
Harris’ gym, the Multnomah Athletic Club, had installed a defibrillator after the American Heart Assn., some 10 months earlier, and the American College of Sports Medicine urged fitness clubs to acquire the devices and train staff members in their use. Within about two minutes, Harris’ heart was shocked back into a normal rhythm. Emergency medical technicians, who had been called immediately, arrived several minutes later.
“I’m here as a survivor, and want [advisory panel members] to realize it doesn’t just happen to older people, or to men or to those who are out of shape: This could be your daughter or your grandchild,” said Harris last week.
Philips estimates that about 70% of those who ask a doctor to prescribe a defibrillator are turned down. Some physicians, said Philips’ scientist Carl Morgan, believe that patients concerned about their hearts would be better off joining a gym or weight-loss program.