2 More Firms Subpoenaed in Dialysis Investigation
- Share via
A federal probe of the kidney dialysis industry heated up Thursday as two more companies, Quest Diagnostics Inc. and Bone Care International Inc., disclosed that they had received subpoenas for documents related to the use of vitamin D.
Four other companies, including DaVita Inc. of El Segundo, received subpoenas this week from the U.S. attorney’s office in New York.
Quest Diagnostics, based in Teterboro, N.J., said it and its test-kit manufacturing subsidiary, Nichols Institute Diagnostics, each received a subpoena for various documents, including those related to tests for parathyroid hormone.
Bone Care International, a Middleton, Wis.-based manufacturer of vitamin D, said the Justice Department had subpoenaed a range of documents, including those related to vitamin D therapies.
Bone Care and Quest said they were cooperating with investigators.
The probe, said to be both criminal and civil, now touches kidney dialysis providers, drug makers and clinical testing labs. Analyst Adam Greene of First Albany Capital said it appeared that “the investigation relates to the billing practices” of kidney dialysis providers.
Medicare, the federal health plan for the elderly and disabled, covers all dialysis patients regardless of age.
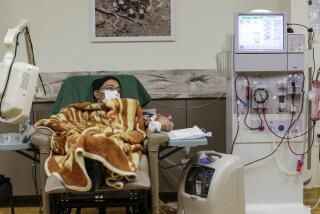
Intravenous vitamin D is routinely given to kidney dialysis patients to control parathyroid hormone, which can cause bone loss. Patients with kidney failure go to clinics run by DaVita and its competitors to have waste removed from their blood.
Vitamin D is profitable for dialysis companies -- Medicare reimbursement is more than 30% higher than the cost of the drug -- creating an incentive for dialysis firms to overuse the medication, analysts have said.
Other firms that have received subpoenas are DaVita competitors Fresenius Medical Care, a German company, Renal Care Group of Nashville and vitamin D supplier Abbott Laboratories of Abbott Park, Ill.
More to Read
Inside the business of entertainment
The Wide Shot brings you news, analysis and insights on everything from streaming wars to production — and what it all means for the future.
You may occasionally receive promotional content from the Los Angeles Times.