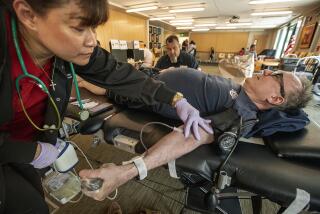
Government revisiting restrictions on blood donations by gay men
- Share via
Reporting from Washington — Federal health officials announced Friday that they would reexamine a 27-year-old set of restrictions on blood donations by gay men.
The restrictions, enacted in the early years of the AIDS epidemic in the United States, impose a lifetime ban on men donating blood if they’ve had sex with another man at any time since 1977.
In recent years, the American Red Cross, the American Assn. of Blood Banks and America’s Blood Centers, which collectively represent almost all blood banks in the country, have recommended loosening the restrictions to allow men who have abstained from gay sex for one year to donate blood.
The American Medical Assn. also has proposed revising the policy but recommended a five-year instead of a one-year waiting period.
Gay rights groups also have pushed for a change in the donor policy, arguing that it stigmatizes gay men and does not adequately address threats to blood safety posed by high-risk heterosexual behaviors.
Changes in the rule have been opposed by hemophilia patient groups. People with hemophilia, a bleeding disorder, are heavy users of blood products, and about 10,000 were infected with HIV in the late 1970s and early 1980s before the current limits were put in place. Thousands of those infected with the virus subsequently died.
Last week, a group of 18 senators, led by John F. Kerry (D-Mass.) wrote to the Food and Drug Administration urging it to revisit the policy on donations by gay men, calling it “outdated, medically and scientifically unsound.”
Improvements in testing technology allow for a revision in the donation rules without threatening the safety of the blood supply, the letter said.
The FDA last examined the donation protocols in 2006 but left the restrictions in place.
The FDA “has been actively engaged in reexamining the issue of blood donor deferral for men who have had sex with other men, taking into account the current body of scientific information, and we are considering the possibility of pursuing alternative strategies that maintain blood safety,” the agency said in a statement.
The issue will be examined by the Department of Health and Human Services’ blood safety committee in June, according to the statement.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.