First trial of vaccine to treat Parkinson’s disease begins
- Share via
The Austrian company AFFiRiS A.G. of Vienna said this week it has begun the first-ever clinical trials of a vaccine to treat Parkinson’s disease. The study of as many as 32 patients is designed to test the safety and tolerability of the vaccine, called PD01A.
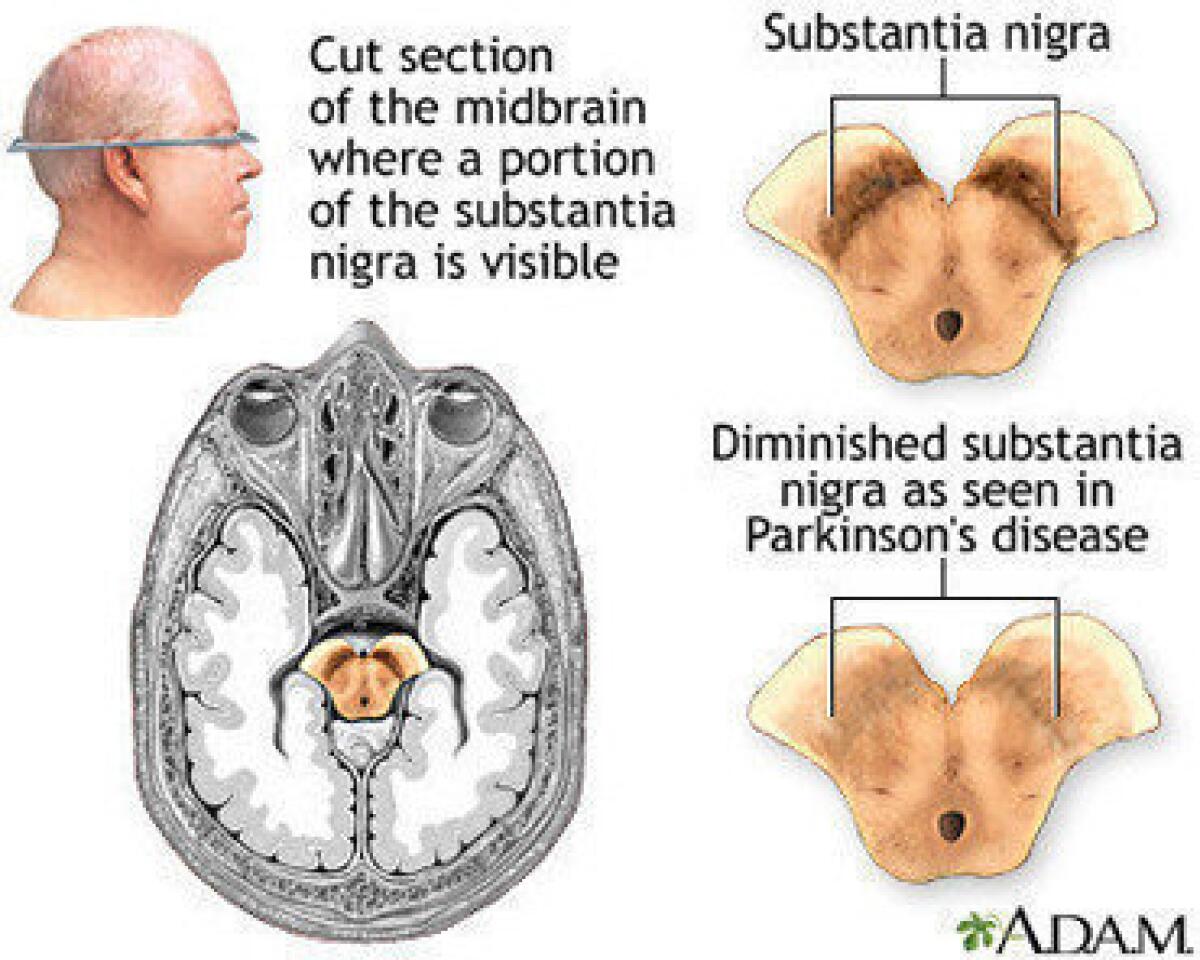
Parkinson’s is thought to result from the deposit of pathological forms of a protein called alpha-synuclein in the brain, causing the death of cells, particularly in the region known as the substantia nigra.
The accumulation of alpha-synuclein disrupts the production of the neurotransmitter dopamine in the brain, impairing movement and causing tremors. The disease is ultimately fatal.
Reducing concentrations of the protein is thought to have a beneficial effect on disease progress. PD01A is designed to stimulate the production of antibodies against alpha-synuclein without affecting closely related proteins.
An estimated 4 million people in the Western world are thought to have Parkinson’s, and that number is expected to increase to 9 million by 2030.
Current treatments involve stimulation of dopamine production or increasing brain cell sensitivity to dopamine, but none of them are targeted at the disease process itself.
The clinical trial is funded in part by a $1.5-million grant from the Michael J. Fox Foundation.
LATimesScience@gmail.com
twitter.com/@LATMaugh