To fight aging, patients experiment with prescription drugs
- Share via
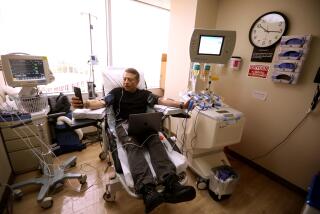
Dr. Alan Green’s patients travel from around the country to his tiny practice in Queens, N.Y., lured by the prospect of longer lives.
Over the last two years, more than 200 patients have flocked to see Green after learning that two drugs he prescribes might stave off aging. One 95-year-old was so intent on keeping her appointment that she asked her son to drive her from Maryland.
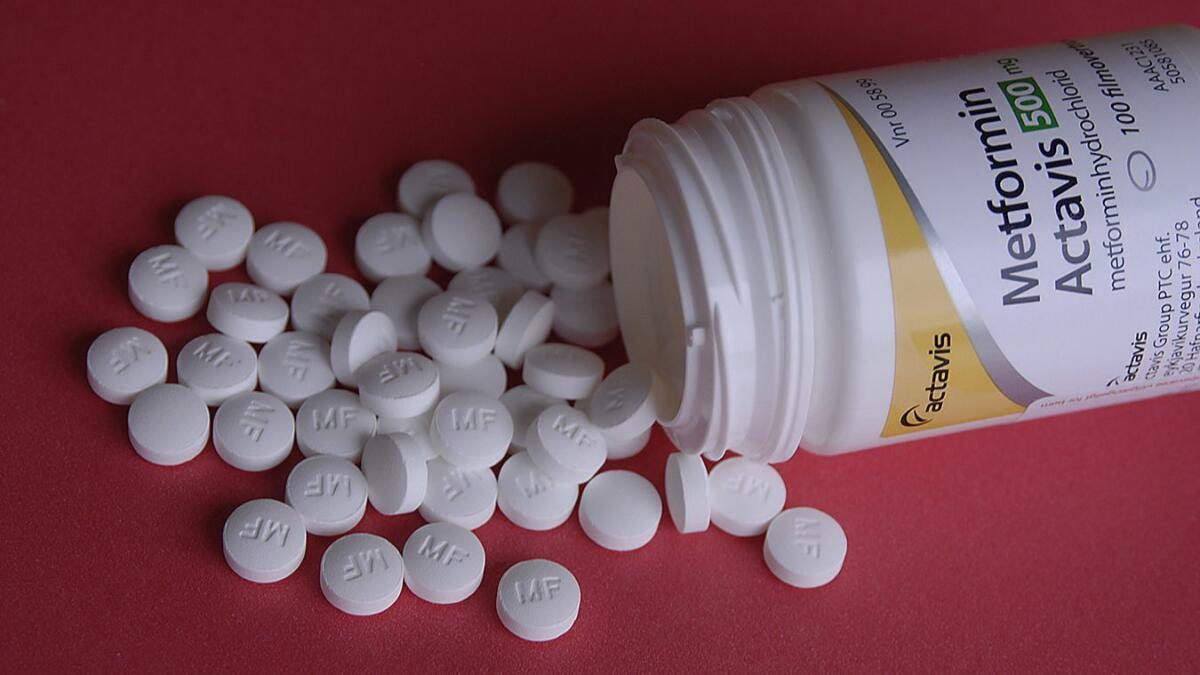
Green is among a small but growing number of doctors who prescribe drugs “off-label” for their possible anti-aging effects. Metformin is typically used to treat diabetes, and rapamycin prevents organ rejection after a transplant. But doctors have the authority to prescribe drugs for any reason they see fit — in this case, for “aging.”
Rapamycin’s anti-aging effects on animals and metformin’s on people with diabetes have encouraged Green and his patients to experiment with them, even though there’s little evidence that healthy people could benefit.
“Many of [my patients] have PhD’s,” said Green, who is 76 and has taken the drugs for three years. “They have read the research and think it’s worth a try.”
In fact, it’s easier for patients to experiment with these drugs — either legally off-label or illegally from a foreign supplier — than it is for researchers to launch a clinical trial that would demonstrate their effectiveness in humans.
No rigorous large-scale clinical trials have been conducted for drugs aimed at fighting aging. The Food and Drug Administration hasn’t even agreed that a treatment could be approved for delaying the onset of aging or age-related diseases, citing questions about whether research can demonstrate an effect on aging overall rather than on a specific disease.
Given such reservations, pharmaceutical companies have little incentive to fund costly trials. Also, both metformin and rapamycin are generic and relatively cheap.
“There’s no profit,” said Matt Kaeberlein, who studies the biology of aging at the University of Washington. “Without profit, there’s no incentive.”
Supplements with purported anti-aging effects routinely enter the market with little scrutiny and less evidence.
Yet, late last year, the National Institutes of Health rejected a $77-million grant proposal for a study to determine whether metformin could target multiple age-related diseases at once. It was the second rejection of the ambitious but unorthodox bid.
“We’re going to keep trying,” said Stephen Kritchevsky of the Sticht Center for Healthy Aging and Alzheimer’s Prevention at Wake Forest School of Medicine, who worked on the metformin proposal. “These things take time.”
Less is known about rapamycin’s anti-aging effects and its possible side effects in the general population, including the possibility it could lead to insulin resistance.
Yet a litany of studies show that rapamycin extends the life spans of animals, and staves off cancer, cardiovascular diseases, cognitive problems and other age-related diseases.
“There should have been a clinical trial for rapamycin and Alzheimer’s disease years ago,” said Kaeberlein, who has urged the NIH to fund studies on the drug’s effects. “But the fact is, the clinical trials are really hard and expensive.”
Alexander Fleming, a former FDA official and advocate for the metformin proposal, said he believed it was difficult for regulators and funders to grasp that aging can be tackled as a whole, not just one disease at a time.
The NIH reviewers who rejected the metformin proposal cited problems with the project’s aim of testing multiple age-related diseases at once. The researchers considered appealing the decision, asserting that those reviewers were biased against studying aging as a whole. NIH, which declined to comment, discouraged the attempt.
Dr. Evan Hadley, director of the National Institute on Aging’s division of geriatrics and clinical gerontology, said projects that target aging are still “of interest.”
The FDA also is open to considering such efforts “based on the scientific evidence presented to us,” said spokeswoman Amanda Turney.
Other investigators have moved ahead with clinical trials focused on specific age-related conditions. For instance, researchers have shown that a “cousin” of rapamycin boosts the effectiveness of flu shots and lowers the incidence of upper respiratory infections in seniors by up to 30%. That group has licensed it from Novartis and is now working on getting approval to target Parkinson’s disease.
At a recent scientific forum on aging, the 300 or so people in attendance were asked to indicate whether they were already taking metformin for aging.
“Half the audience raised their hands,” recalled Dr. Nir Barzilai, director of the Institute for Aging Research at the Albert Einstein College of Medicine.
Barzilai sees metformin as a promising anti-aging treatment, but he’s also concerned about the trend toward off-label prescribing.
“Much of the aging field is charlatans,” Barzilai said. “They tell you take this or that and you’ll live forever. But you have to do a clinical trial that is placebo-controlled, and only then can you say what it really is and whether it’s safe.”
Green said he plans to continue prescribing off-label drugs. He estimates about 5% of his patients are doctors themselves; others have backgrounds in science.
Some doctors who are open to prescribing metformin are holding off on rapamycin, which has caused side effects when given in higher doses to sick patients.
“I need to see more evidence,” said Dr. Garth Denyer, a doctor in the wealthy Houston suburb known as the Woodlands. “I’m hoping to see more data on safety.”
Michael Slattery, who has been HIV-positive since 1983, said he is taking both metformin and rapamycin because the virus is likely to shorten his life. “I feel I have nothing left to lose,” said the retired biotech consultant.
So far, he has not noticed any side effects or benefits. But his partner, who is also HIV-positive, stopped taking rapamycin after getting kidney infections.
Other patients remain hopeful, even though the evidence is unlikely to be definitive anytime soon.
Linda Mac Dougall of Port Hueneme said she participated in a small study that lacked a placebo. She’s uncertain whether it had any effect.
“I really haven’t noticed anything, but that doesn’t mean it didn’t work,” said the 70-year-old massage therapist for seniors. “If I live until I’m 110, we’ll know.”
Taylor is a senior correspondent for Kaiser Health News, an editorially independent publication of the Kaiser Family Foundation.