Story So Far: Superbug outbreaks: What we know about scopes linked to deaths
- Share via
The Food and Drug Administration is holding hearings Thursday and Friday about a medical scope that has been linked to deadly superbug outbreaks in the U.S. and Europe in recent years, potentially sickening hundreds of people. At UCLA's Ronald Reagan Medical Center, three patients died in recent months.
The duodenoscopes have proved difficult to clean of dangerous bacteria such as CRE, or carbapenem-resistant Enterobacteriaceae. That superbug is highly resistant to antibiotics and can kill up to half of infected patients.
Olympus Corp., the leading manufacturer of these specialty scopes, has come under fire for not warning U.S. medical providers sooner about the risk of infection even when its cleaning protocols are followed. The company sent an alert and revised instructions to European hospitals in January 2013 after an outbreak in the Netherlands.
Here's a guide to the scopes, what has happened and what's to come.
What are the scopes used for, and how can they transfer bacteria?
The scopes linked to the outbreaks are long, flexible instruments that are placed down a patient's throat during a procedure known as ERCP, or endoscopic retrograde cholangiopancreatography. The procedure is meant to diagnose and treat problems such as blockages in the bile duct, gallstones and tumors.
The devices have an elevator channel at the tip that can sometimes trap contaminants. The instruments are supposed to go through a lengthy process of cleaning by hand and often another round of disinfection in an automated machine.
Some hospitals that cleaned the device according to manufacturers' instructions, however, still experienced superbug outbreaks.
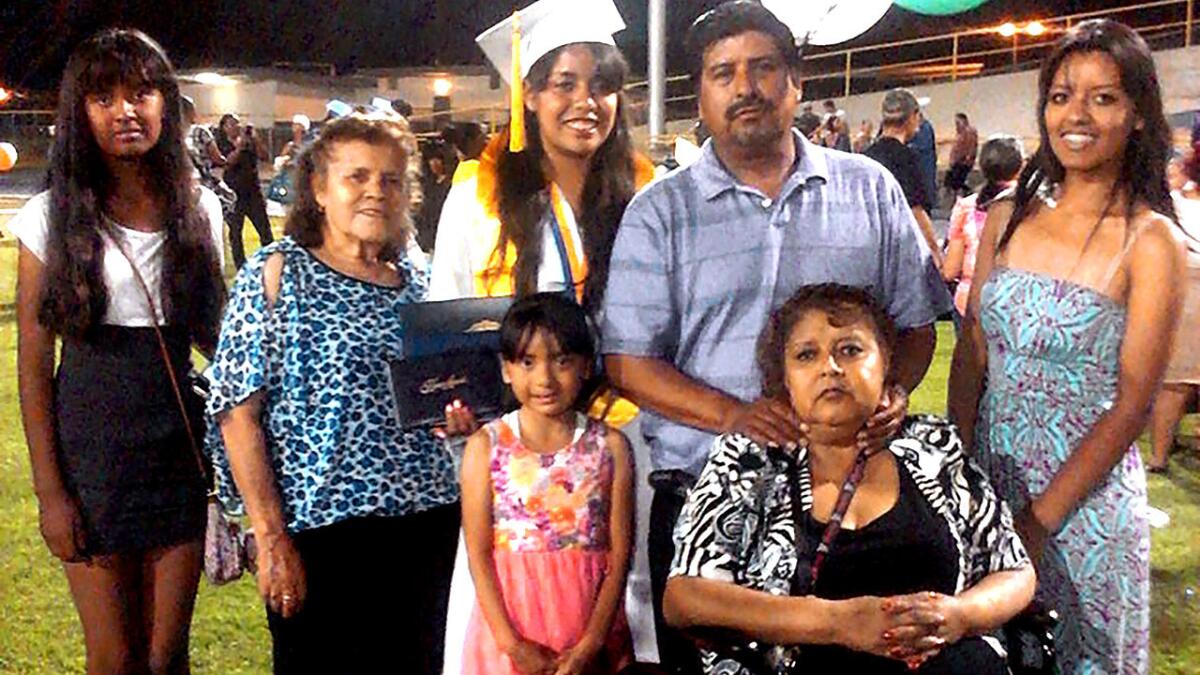
A June 2014 family photo shows Antonia Torres Cerda, bottom right, who died Nov. 8 at UCLA after contracting an infection blamed on an Olympus scope.
Where and when did outbreaks occur?
Federal officials have said they're aware of nine recent outbreaks linked to the scopes at hospitals from Illinois to Seattle. News of the troublesome scopes gained national attention this year, when the Los Angeles Times revealed that seven patients at UCLA were infected with CRE and more than 170 others may have been exposed there. UCLA recently updated that figure to eight infections in total.
In January, it was disclosed that more than 30 patients were sickened by another superbug linked to scopes at Virginia Mason Medical Center in Seattle from 2012 to 2014. Eleven of those patients died, though other factors may have contributed to their deaths.
Outbreaks at hospitals in the Netherlands and Germany predated some of the U.S. incidents. Twenty-two patients became ill at a Rotterdam hospital in the Netherlands in 2012, and in a separate outbreak in Germany, a dozen patients became ill with the same bug that infected UCLA's patients.
How quickly did Olympus and the FDA react?
Olympus, the worldwide leader in gastrointestinal scopes, has drawn the ire of federal lawmakers, hospitals and patient-safety advocates for not taking action sooner to address a well-documented problem with its device.
The company issued revised cleaning procedures to U.S. customers in March 2015, more than two years after taking similar action in Europe.
The FDA has also been aware of potential problems with ERCP scope safety, but it did not publish an urgent alert until after The Times reported on the UCLA outbreak.

Federal regulators issued a nationwide safety alert in February after news broke about a superbug outbreak at UCLA’s Ronald Reagan Medical Center. Above, UCLA officials discuss the scope-related infections.
Federal regulators issued a nationwide safety alert in February after news broke about a superbug outbreak at UCLA's Ronald Reagan Medical Center. Above, UCLA officials discuss the infections. (Anne Cusack / Los Angeles Times)
The FDA has opted against a recall of duodenoscopes, saying such a move could do more harm to the public by taking away a potentially lifesaving procedure. The agency recently said it has received 142 reports of possible patient infection and ERCP scope contamination since 2010.
U.S. Rep. Ted Lieu (D-Torrance) has also blasted Olympus for profiting from the crisis. Some hospitals have adopted more time-consuming measures to clean and test the scopes, so in order to have the necessary equipment on hand, they have bought more of the scopes from the company.
What might happen at this week's hearing?
The FDA has scheduled a two-day meeting for Thursday and Friday to discuss what more can be done to protect the public from scope-related infections. The agency has invited the manufacturers -- as well as doctors from hospitals that have had outbreaks, including a physician from UCLA -- to speak.
At the meeting’s conclusion, the agency will ask a panel of outside experts for their opinion on additional measures to take.
READ MORE: Hospitals step up search for best scope-cleaning protocol
Times staff writers Chad Terhune, Melody Petersen and Geoffrey Mohan contributed to this report.
Follow @JamesQueallyLAT for breaking news
Inside the business of entertainment
The Wide Shot brings you news, analysis and insights on everything from streaming wars to production — and what it all means for the future.
You may occasionally receive promotional content from the Los Angeles Times.








