Stem cell method finds cure
- Share via
Taking the next step in a series of breakthrough stem cell experiments, scientists have cured sickle cell anemia in mice by rewinding their skin cells to an embryonic state and manipulating them to create healthy, genetically matched replacement tissue.
After the repaired cells were transfused into the animals, they soon began producing healthy blood cells free of the crippling deformities that deprive organs of oxygen, scientists from the Whitehead Institute for Biomedical Research in Cambridge, Mass., and the University of Alabama at Birmingham reported Thursday.
“It really works beautifully,” said Kathrin Plath, a researcher at the Broad Center of Regenerative Medicine and Stem Cell Research at UCLA, who wasn’t involved in the study.
The experiments, published online by the journal Science, confirmed the therapeutic potential of a new class of reprogrammed stem cells, which can be custom-made for patients without creating and then destroying embryos.
“This is a platform for any one of dozens of human genetic blood diseases, not just sickle cell anemia,” said Dr. George Q. Daley, a stem cell scientist at Harvard Medical School who wasn’t involved in the research.
The strategy should work to treat hemophilia, thalassemia and severe combined immunodeficiency disease, the so-called bubble boy disease, Daley said. He and others said it would also apply to disorders linked to mutations in a single gene, such as muscular dystrophy and cystic fibrosis.
Scientists ultimately hope to use a similar approach to create cardiac cells to treat heart attack patients or nerve cells that could cure spinal cord injuries. Finding an abundant source of stem cells that could be used as a personalized biological repair kit is the ultimate goal of regenerative medicine.
The technique is still at least a few years away from being used to treat people, scientists said. Before it could even be tried, several rounds of animal experiments would need to be done.
Researchers will also need to overcome some key technical hurdles, including finding a way to reprogram adult cells without using genes and viruses that could cause cancer.
But as a proof of principle, the study is sure to lure more researchers into studying the new class of induced pluripotent stem cells, or iPS cells.
“There’s going to be this tsunami,” said Paul J. Simmons, director of the Center for Stem Cell Biology at the University of Texas Health Science Center in Houston. “One would have to predict that the pace of observations made using iPS cells is going to rise exponentially.”
The study is the latest in a string of significant experiments published in the last five months involving a new approach of reprogramming adult cells so that they are capable of growing into any type of tissue in the body. They have captivated researchers, ethicists and politicians looking for an alternative to embryonic stem cells, which can be difficult to work with and are fraught with ethical problems.
Japanese researchers pioneered the new method, which involves turning on four genes that are dormant in adult cells but active in days-old embryos. Once those genes are activated, the cells essentially forget that they have become skin cells, and they then behave like embryonic stem cells. Because they are derived from a patient’s own cells, there is no risk of tissue rejection.
In June, three research teams showed that the technique worked reliably in mice. Last month, two groups demonstrated that it also worked with human cells. But it remained to be seen whether the cells could serve as the raw material to grow replacement parts for patients.
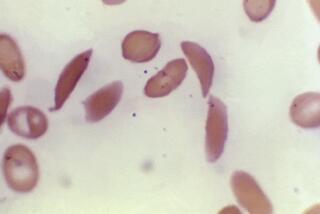
The researchers started with sickle cell anemia because it has a simple origin -- at a key point on the hemoglobin beta gene, patients have what amounts to a misspelling in the chemical letters of DNA, commonly known as A, C, T and G. Instead of having at least one A, they have a pair of Ts. As a result, the gene makes the wrong amino acid, resulting in red blood cells that are curved instead of round.
Those sickle-shaped cells clog up as they travel through the body, blocking blood flow to the small vessels that feed the brain, kidneys and other organs. Tissues die because sickle cells can’t deliver enough oxygen to keep them healthy.
Some patients can be treated with a bone-marrow transplant, which allows the body to make normal red blood cells. But only about 5% of sickle cell patients are able to find a donor, said Dr. Timothy M. Townes, chairman of the department of biochemistry and molecular genetics at the University of Alabama and one of the study’s senior authors.
Townes figured that embryonic stem cells might help the 95% of patients who couldn’t find donors. But the process would be complicated.
First, scientists would have to clone embryos using the patient’s own DNA. Then they would switch one of the errant Ts to an A. Stem cells would then have to be harvested from the modified embryo and used to make healthy bone marrow for a transplant.
But before scientists were able to do that, the first paper on reprogrammed iPS cells appeared.
Townes teamed up with Rudolf Jaenisch, a stem cell researcher at Whitehead and MIT, to see if iPS cells would work in place of embryonic stem cells.
They took cells from the tail of a 12-week-old mouse with sickle cell anemia and used viruses to turn on four dormant genes that are active in days-old embryos. One of those genes, c-Myc, has a tendency to cause tumors, so after the cells had completed their transition back to an embryonic state, the researchers deleted it.
Then they corrected the genetic flaw that causes sickle cell anemia by engineering a string of DNA that had an A in place of a T but was otherwise identical to the original. It was swapped into place with the help of an electric shock.
The researchers grew the iPS cells into bone marrow stem cells by exposing them to special growth factors and culture conditions. When the cells were ready, they were transplanted into three sick mice that were genetic twins of the donor mouse.
Twelve weeks later, the mice were producing the normal version of hemoglobin beta protein, and virtually all of their red blood cells were round. Their body weight and respiratory capacity improved. Their urine, previously watery due to the disease, had normal levels of electrolytes.
None of the mice developed tumors, a sign that the threat from c-Myc had been eliminated.
Plath said it was encouraging that the skin cells could be reprogrammed, genetically altered and able to yield their therapeutic benefits in a relatively short period of time.
“If this is ever applied to the human system, you need this to work fairly fast,” she said. “You can’t waste three years waiting for the cells.”
Jaenisch is now using the same approach to treat other diseases, though he declined to say which ones.