Heart Patient Critical After Enduring 2nd Transplant
- Share via
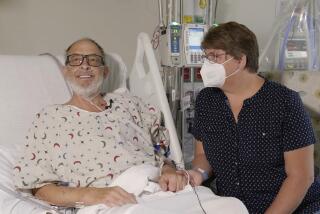
A Huntington Beach man who is in critical condition at UC Irvine Medical Center after his second heart transplant would have a greater chance of survival if the hospital had been permitted to use more sophisticated heart pumps, his surgeon said Friday.
Hospital officials are seeking approval to use state-of-the-art pneumatic pumps, Dr. Richard Ott said. Only 19 American hospitals are authorized by the Food and Drug Administration to use the pumps.
It was the lack of approval--not the $300,000 installation cost--that prevented the hospital from using pneumatic pumps during the second heart transplant surgery for 26-year-old Scott Headding, said Ott, who heads UCI’s heart transplant program.
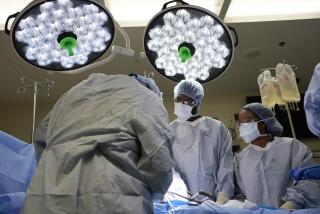
Doctors instead implanted centrifugal-assist pumps to help the heart that Headding received Tuesday to pump blood. But the centrifugal pumps, which have a greater potential for adverse side effects, caused extensive damage to Headding’s liver and kidneys, Ott said.
1st County Transplant
“The same devices that have salvaged his life have threatened his life,” Ott said. “I have difficulty understanding his capacity to survive. He has already beaten insurmountable odds.”
In April, Headding, a musician, became the first person to undergo a heart transplant at an Orange County hospital. The operation received widespread attention because Headding was given the heart of a Marine sergeant who died after a fight with Cal State Fullerton football players outside a bar April 7.
But while the Marine, Richard William Bottjer, could have lived a healthy life with the heart, Ott said, Headding was unable to compensate for the organ’s abnormally thick-walled left chamber. That caused his new heart’s right chamber to fail and forced the second heart transplant. Hospital officials refused Friday to disclose the identity of the second organ donor.
Doctors removed the centrifugal pumps Friday afternoon because of the damaging side effects. A hospital spokeswoman said the surgery went well and that Headding’s heart was pumping blood on its own.
“His (new) heart has recovered dramatically,” Ott said, adding that severe damage to Headding’s liver and kidneys is what now threatens his life.
Asked to assess Headding’s chances for survival, Ott said, “There is always the possibility for difficulties and profound problems.” Without the transplant, doctors had given Headding, who suffers from a degenerative disorder called cardiomyopathy, only a 10% chance of survival.
The only hospital in Southern California authorized to use the pneumatic pump is UC San Diego, a major transplant center. In addition to their use with transplant patients, the centrifugal and pneumatic pumps are used for other types of heart patients.
Like UCI, Hoag Memorial Hospital is seeking approval to use the new pump. Ott and Douglas Zusman, co-director of Hoag’s transplant program, said they believe the FDA will hold the number of hospitals approved to use the new pump to 20.
Competition for Approval
“There has been some competition for that last spot,” Ott said.
William Griggs, an FDA spokesman in Washington, said Friday that he was unable to comment on the approval issue.
Doctors at UCI and Hoag said the pneumatic device is preferable to the older centrifugal pump for several reasons. Chief among them is that the pneumatic device is less likely to break down red blood cells and cause liver and kidney failure. The new pump is made of material more closely resembling human tissue and is more compatible with the body’s organs.
“Yes, it is important to have the (pneumatic) devices,” Zusman said. “But the percentage of your transplant population that needs them is a small percentage.” The percentage of heart patients who need an assist pump is 5% or less.
Hoag is apparently further along than UCI in completing the preliminary steps toward use of the device. Zusman said both he and his partner, Dr. Aidan Raney, have traveled to Salt Lake City for training in use of the pneumatic device. Salt Lake City is home to Symbion, one of the pump’s manufacturers, and the University of Utah, which has pioneered use of the device.
A team from UCI will fly to Utah later this month for similar instruction, Ott said.
Besides FDA approval, UCI and Hoag must be certified by Symbion to use the pump.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.