HEALTH CARE
- Share via
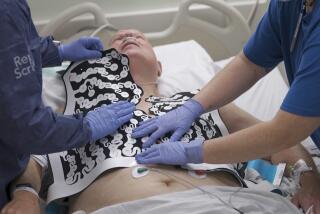
Lifesaving Device: Cardiac Science Inc. in Irvine, meanwhile, is pushing ahead with its development of a medical device that company officials hope will one day save as many as 400,000 lives a year.
The device, called an automatic defibrillator, is designed to deliver a shock to a patient’s heart automatically when an on-board computer senses the onset of cardiac arrest. The therapeutic shocks can be administered in seconds, rather than minutes.
Even in hospitals, patients stricken by sudden cardiac death--a condition in which the heart speeds up uncontrollably and then stops--can die because response with a conventional defibrillator can take as long as 10 minutes.
The first phase of human clinical trials began in February at UC Irvine Medical Center in Orange. The U.S. Food and Drug Administration has given the green light for Cardiac Science to expand its testing to three other medical centers: Albert Einstein College of Medicine in New York, Arizona Heart Institute in Phoenix and New England Deaconess Hospital in Boston.
Two other phases are expected to begin later this year, said Howard Cooper, Cardiac Science’s chief executive. The company hopes to file its formal application for approval by the end of the year, Cooper said, and receive clearance to market the product by 1994.
More to Read
Inside the business of entertainment
The Wide Shot brings you news, analysis and insights on everything from streaming wars to production — and what it all means for the future.
You may occasionally receive promotional content from the Los Angeles Times.