Trial Vaccine Cuts Meningitis Among Children
- Share via
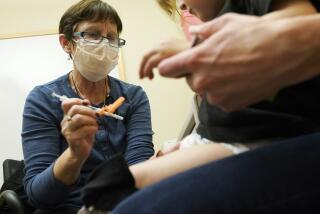
An experimental vaccine has proved 100% successful in preventing bacterial meningitis and severe bloodstream infections in children under the age of 5, researchers said Friday in San Diego.
The vaccine against pneumococcal disease proved so effective that researchers ended a three-year trial prematurely and immunized all of the 38,000 children being studied, a team from the Kaiser Permanente Vaccine Study Center in Oakland told a meeting of the Interscience Conference on Antimicrobial Agents and Chemotherapy.
“This is a big win for children,” said Dr. Jerome Klein of the Boston University School of Medicine, a leading expert on pneumococcal disease. “Meningitis will now become an uncommon disease.”
The vaccine could be widely available as early as next year.
Currently, pneumococcal infections can be treated with antibiotics, but many infections are not caught in time to prevent complications. Physicians also fear that pneumococcus, like other bacteria, will become increasingly resistant to existing antibiotics.
In the United States, about 10,000 children develop pneumococcal infections each year, of which roughly 1,500 are meningitis--an inflammation of the brain and spinal cord--while the rest are blood infections. About 300 children a year die of meningitis--including a 13-year-old Orange County girl who died earlier this week. Her infection, however, was caused by a different type of bacterium which would not be prevented by the new vaccine, said an official of the Orange County Health Care Agency.
Many of those who survive meningitis lose their hearing, have seizures or incur developmental problems. Worldwide, more than 1.2 million children die of pneumococcal infections annually.
“In my mind, this is the biggest vaccine breakthrough in the past eight to 10 years,” said Dr. Henry Shinefield of the center, one of the co-leaders of the trial.
The team hopes the vaccine will also help protect against the 7 million ear infections (otitis media) each year caused by pneumococcal bacteria, but results from that portion of the study will not be available for a month.
Pneumococcal disease is actually a group of illnesses caused by the bacterium Streptococcus pneumoniae, often known simply as pneumococcus. These illnesses include severe, invasive infections such as bacteremia (infection of the blood) and meningitisas well as pneumonia (infection of the respiratory system), otitis media and sinusitis.
Including adults, pneumococcal disease causes about 40,000 deaths in the United States each year, and is responsible for 3,000 cases of meningitis, 50,000 cases of bacteremia and 500,000 cases of pneumonia.
There is already an effective vaccine against pneumococcal infections, but it does not work in children under the age of 2. Their immune system does not respond to the bacterial proteins used in that vaccine, Klein said.
The new vaccine uses technology similar to that employed in the Hemophilus influenza type b, or Hib, vaccine introduced nearly 10 years ago. There were 20,000 cases of bacteremia and meningitis caused by Hib every year among children before that vaccine was introduced--about 60% of the total number of such illnesses.
Shinefield and Dr. Steven Black enrolled more than 38,000 children in the study at 23 Kaiser Permanente sites throughout Northern California. Half got the new vaccine at the ages of 2 months, 4 months and 6 months, while half got a placebo vaccine at similar intervals. Neither patients nor physicians knew who received the actual vaccine.
By August, 22 children had developed pneumococcal bacteremia or meningitis. When Shinefield and Black “broke the code” to learn which patients had received vaccine, they found that all the cases were among the children receiving a placebo. They immediately halted the trial and began immunizing all the children.
Four more cases have subsequently been discovered, again among the placebo group. The vaccine thus proved 100% effective.
“You don’t see many vaccine trials where the results are that dramatic,” Klein said.
But experts cautioned that the vaccine will not prevent all infections. About 80 strains of the bacterium are known, and the vaccine protects only against the seven most common strains. “It will have a substantial impact, but it won’t wipe out all pneumococcal disease,” Klein said.
The vaccine’s manufacturer, Wyeth Lederle Vaccines, said it would apply to the Food and Drug Administration later this year for permission to market the vaccine.