Drug-Resistant Germs Get New Foe
- Share via
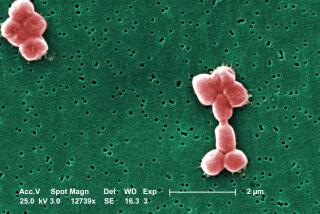
WASHINGTON — The Food and Drug Administration on Tuesday approved the first in a new class of antibiotics capable of fighting several different strains of drug-resistant bacteria, including a common infection called Staphylococcus aureus that public health officials regard as an increasingly worrisome bug.
Zyvox, made by Pharmacia & Upjohn of Kalamazoo, Mich., is the second new antibiotic in recent months to be licensed by the FDA. Like Synercid, which was approved in September, it offers another weapon against the growing threat posed by drug-resistant microbes.
Zyvox, however, is the first drug in 40 years to be developed and approved specifically for infections caused by staph that fail standard antibiotic therapy.
The drug works against certain common bacterial pneumonias and skin infections that often afflict patients in hospitals and can prove deadly. These include infections that fail to respond to all available antibiotic treatments--such as vancomycin, regarded until recently as the drug of last resort.
Staph is commonly found on the skin of healthy people and usually causes no harm unless it enters the body. When it does, it usually can be treated effectively with the first-choice antibiotic, methicillin. When methicillin does not work, vancomycin is used. Zyvox will now provide an alternative.
In recent years, however, public health officials have become anxious about the sudden appearance of staph infections that fail treatment with vancomycin.
Since 1997, there have been only about eight reported cases of vancomycin-resistant infections in hospitals. But, based on their experience with bacteria resistant to other antibiotics, experts fear that number will grow sharply.
Experts are hopeful that Zyvox also will prove effective against these infections because it has shown promise in the laboratory. But with so few cases, they are not sure.
“It [staph] is the organism that everybody in public health is extremely concerned about, so when new products are developed, we hope they will be useful for treating these infections,” said Dr. Gary Chikami, director of the FDA’s anti-infective drug products division.
Zyvox also joins Synercid--which is actually a potent combination of two existing drugs--in fighting vancomycin-resistant Enterococcus faecium, an infection that can be deadly to hospital patients, and other infections caused by difficult-to-treat bacteria.
Health officials estimate that more than 20% of hospital-acquired Enterococci infections--as many as 20,000 annually--are now resistant to vancomycin, a rapid rise since the first such case was reported in 1989. They are worried that vancomycin-resistant staph infections will proliferate in the same manner.
Unlike other antibiotics, the new drug attacks bacteria at a very early point in the reproductive process. It works by stopping the production of protein. Without the ability to produce protein, bacteria cannot multiply--and they die.
“It appears to act differently than other antibiotics that inhibit protein synthesis, which is good news in that the effect of the drug is on a totally new target,” said John La Montagne, deputy director of the National Institute of Allergy and Infectious Diseases, part of the federal government’s National Institutes of Health.
“Zyvox has a profile that makes it truly unique,” agreed Dr. Robert Moellering, professor of medicine at Harvard University Medical School. “It represents an important discovery.”
The drug produces only mild side effects, unlike Synercid, which can be “quite toxic,” according to Dr. Nancy Madinger, an infectious diseases specialist at the University of Colorado Health Sciences Center.
Since Zyvox can be taken in oral form as well as intravenously, the drug should continue to help patients once they leave the hospital.
“I’m very excited about it,” she said. “We need new classes of antibiotics desperately.”
Also, because Zyvox has not been used for treating infectious diseases, it may have an advantage over other antibiotics because bacteria might be slower to develop resistance to it.
Drug-resistant bacteria have become a growing concern in recent years, in part because of the indiscriminate use of antibiotics. Over-exposure to drugs allows bacteria to develop resistant genes. Because bacteria evolve quickly, a few resistant microbes can become millions literally overnight.
Because of this, the FDA has recommended that use of the drug be limited--at least at first--to patients in hospitals or other institutions, to avoid overuse, which could lead to resistance.
The drug--expected to become available within several weeks--was studied in several clinical trials involving more than 4,000 patients, the FDA said.