Over-Counter ‘Morning-After’ Pill Urged
- Share via
ORLANDO, Fla. — Stepping into a morally charged debate Tuesday, the American Medical Assn. called on the Food and Drug Administration to consider making the “morning-after” pill available over the counter.
The AMA’s policy-making House of Delegates approved the resolution without discussion during a convention here. The AMA has 293,000 doctors as members.
“This is a wonderful decision by the AMA. This is a terrific resolution,” said Joan Coombs, senior vice president of Planned Parenthood. She said widespread use of the morning-after pill could prevent 1.7 million unplanned pregnancies and 800,000 abortions annually.
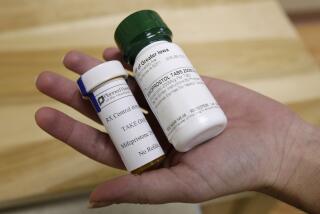
Taken within three days of sexual intercourse, the prescription morning-after pill prevents ovulation or, if that has already occurred, blocks implantation of a fertilized egg in the uterus. AMA members suggested that some women might not be able to get the pill in time to prevent a pregnancy unless they are made available over the counter.
The morning-after pill is essentially a high-dose birth control pill. It is different from the RU-486 abortion pill, which acts by causing contractions to expel an embryo from the uterus. RU-486 can be given up to seven weeks after the start of the last menstrual period.
There are two morning-after pills on the market: Preven and Plan B. They were approved for U.S. use within the last two years.
For the FDA to make them available over the counter, a pharmaceutical company would have to submit an application. The FDA would take into account such things as the drug’s written instructions and its safety history.
FDA spokeswoman Susan Cruzan would not disclose whether the makers of the morning-after pill have applied for over-the-counter sale.
The maker of Preven, Gynetics of Somerville, N.J., has not yet applied to the FDA, said Chief Operating Officer Jack Stover. He added that Gynetics is looking to form a partnership with a bigger company. The manufacturer of Plan B, Women’s Capital Corp. of Bellevue, Wash., is working on its application to the FDA, company founder Sharon Camp said.
The Vatican recently condemned the morning-after pill, and the nation’s largest retailer, Wal-Mart, decided last year not to offer it at its 2,400 pharmacies, saying it might not sell well enough to make it worthwhile.
Coombs said that if the FDA makes the drug available over the counter, “it will make it more acceptable and consumers will demand it.”
Although the morning-after pill is not as widely opposed as RU-486, opponents still consider it a form of abortion.