No Obscure Expertise
- Share via
Long before anthrax spores killed a man in Florida, infected several other people and set off scares and brisk Cipro sales across the country, a handful of scientists worked in relative obscurity to grasp how the tiny bacterium wreaks its havoc on the human body.
They have made big strides. They have almost finished determining the genome structure of Bacillus anthracis . They have learned a lot about how the microbe’s toxins work and how the bacterium can craftily evade destruction by human immune cells. They know something too about genes that render mammals (presumably including humans) more resistant to the onslaught of the microbe.
And, as their understanding has grown, so have ideas for new therapies and vaccines.
Better therapies are sorely needed in the face of a bioterrorism threat, experts say, because the inhalation of anthrax--the rarest but most serious mode of infection--is usually lethal if not treated very early with antibiotics. If too much time passes, a patient’s body is flooded with enough toxins to kill even after the actual bacteria are gone.
Better vaccines are needed too. The ones used in this country are either considered too risky for humans and only used for livestock or, as in the vaccine available only to the military, has side effects and a daunting injection regimen: six jabs over 18 months.
It can take years to translate basic lab research into therapies, but the National Institutes of Health is trying to speed up anthrax trials, said Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases.
“We are meeting with investigators in the next few days to try and expedite the translation of these important findings made in animals and test tubes into early trials in human subjects,” Fauci said last week.
Successful new therapies would help fight a bacterium that has long troubled humans, albeit mostly by infecting their livestock.
The Bible’s descriptions of the fifth and sixth plagues that hit Egypt may be accounts of an ancient outbreak of anthrax, first in animals then spreading to people as boil-like skin infections, some experts believe.
The anthrax might have spared the Israelites because their sheep would have been grazing on poorer pastures where infections don’t take hold as well. “I’ve seen countless outbreaks like that,” said epidemiologist and anthrax expert Martin Hugh-Jones of Louisiana State University.
B. anthracis also played a big part in the history of medicine, as the first-ever bacterium shown to cause disease. In 1877, pioneering microbiologist Robert Koch cultured the bacillus in his lab then injected it into animals and showed that the animals contracted anthrax. Soon after, medical giant Louis Pasteur developed a crude anthrax vaccine.
Yet despite its long history, some scientists suspect B. anthracis is a relatively new microbe on the block--evolved from bacteria that live and divide quietly in soil.
That might explain why cultures of the microbe collected from far-flung places around the world don’t vary much genetically. It also may explain why B. anthracis’ DNA is so similar to that of its closest relatives, said Tim Read, principal investigator of the nearly completed effort to determine the exact structure of the B. anthracis genome at the Institute for Genomic Research in Rockville, Md.
If you look at the bulk of the anthrax microbe’s DNA, “it looks very much like your standard soil bacterium,” Read said.
But there are a few crucial genes that make B. anthracis far from benign. They’re housed on two large rings of DNA analyzed in a separate effort by scientists at Los Alamos National Laboratory in New Mexico.
One DNA ring carries genes that make the bacterium’s lethal toxin. Another carries genes for the creation of a slippery outer coat (or capsule) that helps the bug elude the immune system.
“These genes came from somewhere--where, that’s the mystery,” Read said.
Having the genome nearly mapped out is proving enormously useful, said anthrax researcher Theresa Koehler, associate professor of microbiology at the University of Texas at Houston. “It’s a huge help to our lab. It has sped up everything that we want to do,” she said.
Already, scientists know a lot. They know that B. anthracis needs genes for both the capsule and the toxin to wreak its havoc. Get rid of the capsule and the infection won’t take. Get rid of the toxin and the infected creature can overcome the bug.
Anthrax toxins are unusual, Koehler said, and they operate with slick efficiency.
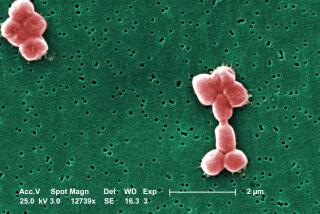
Three proteins are involved in making the two toxins found in anthrax. One toxin protein, called edema factor, causes swelling of infected tissues. Another toxin protein, called lethal factor, is far more serious, killing the cells it invades in ways that are still unclear.
But first, the toxins have to get into cells, a job accomplished by a third protein. Many copies of this third protein attach themselves to a human or animal cell and clump together in groups of seven. The toxins then come in and attach to the clumps.
Next, the cell unwittingly engulfs the whole structure in a bubble. Through a series of steps, the clump of seven proteins changes its shape and pokes a hole through the wall of the bubble. The toxins can then enter the cell and kill it.
“It’s quite elegant, actually, in a hideous sort of way,” said John Collier, professor in the department of microbiology and molecular genetics at Harvard Medical School.
Collier says he started studying anthrax out of academic interest at a time when B. anthracis was hardly the subject of water cooler talk throughout the country.
But the work he’s done with his colleagues on anthrax toxins have led him to potential therapies.
In one approach, he and co-workers subtly altered the protein that attaches to cells. The altered form still attaches and forms those clumps, but it can’t poke a hole in the bubble membrane. “The whole thing is inactive, dead,” Collier said.
Theoretically, if a person is infected with anthrax, doctors could inject the altered protein to interfere with the toxins, preventing them from entering cells. “The hope is that you might rescue somebody at a later stage of anthrax than is now possible with antibiotics,” Collier said.
The same protein also might act as a vaccine: it’s the same one used in a current anthrax vaccine but in a much purer form. The hope, Fauci said, is it could be given to soldiers and others at risk in larger amounts and fewer times than the vaccine now used--and with fewer side effects.
Collier and colleague George Whitesides, a Harvard chemist, have designed another drug that uses a different approach to block the anthrax toxins from getting into cells. Both approaches protect rats from lethal doses of toxins.
The next step is seeing whether the drugs protect animals against an actual anthrax infection.
Other efforts to find better vaccines are underway, as well as studies into the microbe’s life cycle that might yield clues to more therapies. If scientists knew the identity of the receptor on human cells to which the anthrax toxins bind, they might be able to design drugs to interfere with the binding.
Historically, anthrax has been a veterinary problem far more than a medical one, and it has not been a widespread problem in the United States for decades. When Koehler set up her lab 10 years ago, “Some people even questioned, ‘Oh gosh, are you going to try to build your career on Bacillus anthracis ? Good luck!”’
Attention increased a few years ago, after revelations that Iraq had stockpiled anthrax spores. Now the calls are pouring in so heavily that anthrax researchers have two options: either don’t answer or be on the phone all day.
“It’s not just the press calling me but all kinds of people with ideas for a new decontamination procedure or a new vaccine, and of course that’s good, that scientists are coming on board,” Koehler said. “But it’s just gone through the roof.”