The Virus as a Tool of Medicine
- Share via
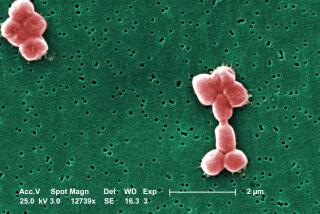
They are many times smaller than a bacterium--yet can easily reduce one to rubble. With steely purpose, they stick to the wall of a bacterial cell and inject their genetic material. Then they use the cell to copy themselves over and over again before crashing out and drifting off to cause more mayhem elsewhere.
Just as there are viruses that infect and cause disease in humans, so there are viruses that infect bacteria.
For decades, researchers in the former Soviet bloc used and studied those viruses--known as bacteriophage, or “bacteria eaters”--in hopes of curing disease. Now, after years of ignoring them, U.S. scientists are showing increasing interest.
The need for new approaches in fighting disease is obvious, says Marissa Miller, who directs the antimicrobial resistance program at the National Institute of Allergy and Infectious Diseases. Overuse of antibiotics has caused drugs to lose a lot of their clout as more and more bugs become resistant.
This is especially a problem in hospitals, where already-sick people can develop complications such as pneumonia and blood infections from bacteria that are resistant to nearly all available drugs.
“In these cases, the therapeutic options are very limited--and usually include drugs that are very toxic,” Miller says.
In response to the problem, scientists and companies are exploring avenues such as small, antibacterial proteins, therapies to boost people’s immune systems--and bacteriophage.
Some are studying specific molecules that the bacteriophage make, in the hope that they might be developed into drugs. Others envision a time when cocktails of bacteriophage that attack various dangerous bacteria might be swallowed or injected or applied to wounds to kill offending microbes.
“It’s an important research area,” says scientist Carl Merril, a bacteriophage expert at the National Institutes of Health. “People are going to have to put some work into it, but it’ll be a real shame not to develop these agents, because I really think they can save lives.”
Most Abundant Life Form on Earth
Bacteriophage--or “phage” for short--are everywhere in nature and are probably big players in the ecology of the microworld. One milliliter of unpolluted water can contain 200 million phage, and there are about 1031--1 with 31 zeroes after it--of them on this planet, making them the Earth’s most abundant life form.
They vary enormously. Phage come in a medley of shapes, from simple spheres to rods equipped with elaborate landing gear that give them the look of alien insects. Their genes can be of RNA or DNA. They have different ways of getting into cells and different consequences: some always destroy their hosts while some hang around in cells for long periods without causing harm.
And while some phage have been studied in exquisite detail, some are entirely unknown.
The first phage were independently discovered around 1915 by two scientists, a British bacteriologist, Frederick Twort, and French-Canadian microbiologist Felix d’Herelle, who was working at the Pasteur Institute in Paris.
D’Herelle--who actively pursued the discovery--had cultured stool samples from soldiers suffering from dysentery and had noticed something odd about the bacteria growing in his petri dishes. The lawn of happily growing Shigella microbes had bullet-like holes in them.
The holes were places where phage had infected cells and killed them.
D’Herelle suspected he was dealing with a virus and began a long series of experiments and treatments. His first patient was a 12-year-old child who was gravely ill with dysentery. D’Herelle and colleagues made a phage preparation, swallowed some of it themselves to make sure it wasn’t harmful, then treated the child and several others. All of the patients survived.
In subsequent years, D’Herelle experimented with phage to treat diseases in chickens as well as human diseases such as cholera and bubonic plague. He also manufactured commercial products containing different types of phage to treat abscesses, infected wounds, respiratory infections and more.
Products in Wide Use Before World War II
For a while, phage nostrums were everywhere. They were manufactured in the United States before World War II. They were even used during the war by the Germans: vials of phage were found in the medical kits of Rommel’s forces in North Africa.
And in the Soviet Union, phage therapy flowered under Joseph Stalin, who helped D’Herelle and scientist Giorgi Eliava set up a huge research institute in Georgia, where tons of phage were manufactured daily. (Eliava was later pronounced a “people’s enemy” and executed.)
In the West, however, phage therapy was dropped entirely--in large part because of the discovery of penicillin and other antibiotics.
“The feeling was--why bother with phage therapy or anything else, once we had those miracle drugs?” says Alexander Sulakvelidze, a scientist at the University of Maryland and co-founder of Intralytix, one of several U.S. companies now developing phage therapy.
Phage were still studied, however--for quite a different purpose. Simple and easy to grow in huge numbers, they were used in practically every landmark experiment in the early history of molecular biology: to show that the blueprint of life was carried in DNA; to crack the genetic code, to cite just two examples.
And it was studies with phage that led to the discovery--quite by chance--of enzymes that allowed DNA to be cut into pieces at will, ushering in the genetic engineering revolution.
Today, increased contact with scientists in former Soviet bloc countries has helped revive an interest in the phage as a medical tool.
Before, “we didn’t talk with them, didn’t know their science,” says William Summers, molecular biologist and phage historian at Yale University School of Medicine.
“Now we’re getting to know them and talk with them. They’ve shown us what they do, and it sounds reasonable.”
Nevertheless, scientists cite problems with many of the phage studies from these countries, some of which involved thousands of people.
The studies rarely contained controls or the double-blind design considered state-of-the-art for clinical trials today--making it hard to know if the phage actually improved patients’ recovery. The brews of phage used were often too diluted, or dead, or inappropriate for the infection being treated.
Recent Studies Show Potential
Recently, scientists have published more rigorous experiments using animal models.
For instance, a series of British studies from the 1980s onward showed that mice, calves and chickens could be protected from disease-causing strains of E. coli, the common stomach bacteria, by treatment with phage that specifically attack the bad E. coli and leave the normal E. coli alone.
In some cases, phage were found to be more effective than antibiotics.
More recent studies have shown that phage can fight particularly troublesome microbes.
In January, Merril at NIH, in collaboration with a company called Exponential Biotherapies, showed that a bacteriophage can protect mice from a particularly dangerous bacterium.
The microbe, vancomycin-resistant Enterococcus faecum, is increasingly being found in hospitals and nursing homes and can cause life-threatening infections of the blood and heart valves.
The company has completed early clinical trials--testing safely in small numbers of people--with the phage.
Companies are pursuing other bugs, too.
Exponential Biotherapies, Intralytix and another company called Phage Therapeutics are variously investigating phage to fight antibiotic-resistant strains of Staphylococcus aureus, multi-drug-resistant tuberculosis, pathogens infecting burns and ulcers, as well as various food-borne illnesses.
In the latter case, food products or animals such as chickens could be treated with phage to kill disease-causing bugs.
Other scientists are working not on the entire phage, but on parts of it.
Vincent Fischetti, head of Rockefeller University’s bacterial pathogenesis lab, has shown that phage enzymes called lysins, which poke holes in a bacterium’s wall, can protect mice from infections.
Each lysin--just like each phage--is extremely picky about the kind of bug it will attack.
“You’re only killing the organism you want to kill,” Fischetti says.
And Ry Young, a bacteriophage researcher at Texas A&M; University in College Station, has found that some phage contain short proteins that are toxic to bacteria and which could conceivably be developed into a new class of drugs to fight infection.
Phage have their disadvantages. Among other problems, doctors have to know precisely what infection they’re fighting in order to know which phage to commit to the battle. And some types of phage carry genes that can actually make bacteria pathogenic. On the other hand, phage do not attack our normal microbial flora--only the disease we want to fight.
Plus, Young points out, “They’re the only medicine that multiplies.”
*
(BEGIN TEXT OF INFOBOX)
life Cycle of a Bacteriophage
(text of the infobox not included)