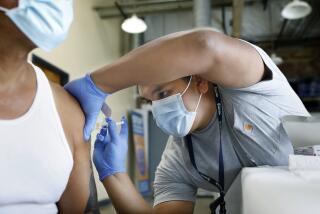
Blood Test to Detect HIV in 20 Minutes Nears Approval
- Share via
Federal regulators are expected to give final approval soon to an easy-to-use finger-prick blood test that can diagnose HIV infection in 20 minutes. The action will come more than four years after public health officials declared the urgent need for such a tool.
People familiar with the approval process say the Food and Drug Administration has resolved lingering technical and manufacturing issues that have held up approval.
AIDS advocacy groups have accused the FDA of dragging its feet while reviewing tests that have been proved extremely accurate and used in 90 other countries. Some of the proposed blood tests are as easy to use as a home pregnancy test or blood sugar monitors for diabetics.
The test proponents say the delays have endangered the public’s health because nearly a third of those who test positive for HIV each year never return to find out their results, which can take as long as two weeks to process. When that happens, infected people go untreated and spread the virus unwittingly to others.
“The potential for saving lives by using this technology, we think, is revolutionary,” said Clint Trout, associate director of federal government affairs for Los Angeles-based AIDS Healthcare Foundation. “We think that the rapid test could be for prevention what protease inhibitors have been for treatment.”
FDA officials declined to comment on their timeline. The agency granted preliminary approval in May to two rapid HIV tests, pending inspections of manufacturing plants and approval for the products’ labeling.
It is not clear when the approval announcement will be made but some people familiar with the process say it could come within the next two weeks.
The first test slated to be approved is OraQuick, manufactured by OraSure Technologies of Bethlehem, Pa., people familiar with the process said.
When the test detects the presence of HIV antibodies, it displays two red bars on a small strip enclosed in plastic. The device is designed for just one use, and the company has not disclosed its price.
With approval near, the debate has shifted to a separate issue that could determine whether the rapid HIV test is widely used.
AIDS activists and public health experts want the federal government to waive requirements that the tests be performed only in technically sophisticated labs.
Though not advocating home use, those advocates say they want the test to provide quick results to sex-club visitors, homeless people at shelters and emergency room patients. Those target populations are among the most likely not to return for their results after they are tested.
Supporters also want to offer rapid tests to late-term pregnant women. If a woman tests positive for HIV before or during delivery, she and her baby can be treated with medications, reducing the chances of transmitting the virus by two-thirds or more.
The military is interested in using rapid HIV tests on the battlefield. When there are many casualties, doctors often request immediate blood donations from fellow soldiers, and there’s little time to test for infections.
Despite these arguments, an advisory panel to the FDA has recommended that the government require the tests be performed at sophisticated laboratories. The panel found that untrained personnel often fail to follow manufacturers’ test instructions and may lack the skills to interpret the results.
“Until it can be demonstrated that individuals performing this test out on the street have a significantly high degree of accuracy in performing the test, the potential danger to the person being tested is simply too high at this point,” said Dr. Jared Schwartz, a pathologist and spokesman for the College of American Pathologists, a medical specialty society that also accredits labs.
Dr. Nelson Michael, a strong backer of rapid tests, disagrees with that reasoning.
“These tests are ridiculously simple,” said Michael, chief of molecular diagnostics and pathogenesis at Walter Reed Army Institute of Research. “You basically defeat that ease of testing if you demand that the test be executed in sophisticated laboratories.”
The Centers for Disease Control and Prevention has been seeking advice from both sides on ways to allow widespread use of the tests while ensuring accurate results, said Dr. Bernard Branson, a medical epidemiologist with the agency. The agency held a conference in Atlanta earlier this month to discuss the topic.
The stakes are high. Each year, more than 20 million people are tested for HIV, including 2 million in publicly funded clinics. Federal health officials estimate that 40,000 people contract the virus each year, and 900,000 people are living with it.
The standard test for HIV, known as ELISA, takes a minimum of five hours to process, and sometimes as long as overnight. But because the test often is sent to labs--where it is run in batches--results often are not available for days or even weeks.
Both with ELISA and the new rapid test, a person may be infected for several months before producing enough HIV antibodies to show a positive result.
The CDC estimates that about 9,600 HIV-positive people do not return to the publicly funded sites for their test results each year, in addition to about 860,000 people annually who test negative but don’t find out. In many cases, test takers are not required to provide their identity or a way to reach them.
“There’s a missed opportunity, both in terms of people being aware of their status and informing their partners,” said Michael Montgomery, director of the office of AIDS within the California Department of Health Services.
A presidential advisory council and even some members of Congress have grown restless that test approval has taken so long.
“We have an FDA that is a bureaucracy that is totally uncontrolled,” said former Rep. Tom Coburn, a physician who chairs the Presidential Advisory Council on HIV/AIDS. “Bureaucrats never take a chance to do something right. They always take a chance to protect their own backside.”
Dr. Elliot Cowan, a senior regulatory scientist at the FDA, said his agency is “working on it as fast as we can.... It would be far worse in my mind, at least, to approve a product and then have it fail once it reaches the marketplace,” Cowan said.
In their defense, federal officials point to British Columbia, Canada, where rapid testing was halted this April because of concerns that test kits provided negative results to people who actually had HIV.
An official at OraSure said he understood the reasons for the lengthy approval process.
“They have a regulatory protocol that needs to be followed, and they are following that,” said Ronald H. Spair, the company’s chief financial officer. “I can’t characterize anyone as stonewalling this.”
Once its OraQuick product is approved, the company wants to conduct studies on a rapid test using oral fluids instead of blood. Other companies want to manufacture rapid tests based on urine.
Technically, OraSure’s product won’t be the first rapid HIV test in the United States. Since 1993, Abbott Diagnostics has sold a test that can provide HIV results within 20 minutes. But advocates and public health experts say the test isn’t very useful because it requires cold storage before use and is labor-intensive to perform.
Abbott has developed an easier-to-use rapid test, called Determine, which is manufactured and widely used outside the United States, but the company has not sought approval to sell it here. It is partnering with OraSure to distribute the OraQuick test here.
MedMira Inc., based in Canada, had expected the go-ahead a year ago. Instead, it received its preliminary approval in May, and officials are still waiting for the green light. The entire research and development staff is now devoted to working with the FDA to answer remaining questions.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.