Smallpox Vaccinations to Proceed Despite Calls to Slow Bush Program
- Share via
WASHINGTON — Government health officials insisted Friday that President Bush’s smallpox vaccination program would move forward as planned, despite a drumbeat of calls to slow down.
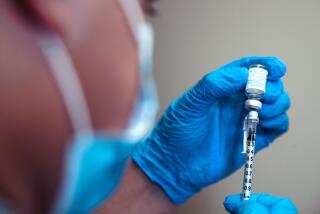
Just hours after a scientific advisory committee urged the government to “proceed cautiously” and one day after labor unions representing 60,000 registered nurses and 750,000 other health workers asked for a delay, officials at the Centers for Disease Control and Prevention announced the “really good news” that 11 states could begin administering the smallpox vaccine as soon as Jan. 24.
The CDC made its announcement about an hour after accumulating requests for shipments of the vaccine from the 11 states. CDC officials refused to identify the states, and in Sacramento a spokesman for the state Department of Health Services said California was not one of them.
“We will ship the vaccine Tuesday,” said Dr. Julie L. Gerberding, CDC director. “This shows that states are ready to go.”
The CDC’s spirited defense of the plan to vaccinate up to 10.5 million health-care workers and police, fire and emergency personnel in preparation for a possible bioterrorism attack using the smallpox virus came in direct response to a report released earlier Friday.
In that report, a committee of the private Institute of Medicine, which was convened at the request of the CDC, urged health officials to move more slowly in implementing the plan, which it called “a program with inherent serious risks and with publicly unknown and unstated benefits.”
The Institute of Medicine committee urged the CDC to more clearly explain the risks and benefits of the vaccine to health workers being asked to volunteer for it. Citing studies showing that one or two of every million vaccinated persons will die and many more will suffer serious complications, the committee said, “The smallpox vaccine may be the least safe vaccine ever used on a wide scale.”
“The reason to give [the vaccine] is for reasons of security and biodefense,” said Dr. Brian L. Strom, a professor at the University of Pennsylvania School of Medicine and chairman of the committee. “The people who take it are taking it for the rest of us ... for the public good.”
The committee also urged officials administering the vaccine program to talk more openly with volunteering health-care workers -- before they make the final decision to be vaccinated -- about whether they would be compensated for lost wages or medical expenses caused by a bad reaction to the vaccine.
Then “it’s up to them whether or not to take” the vaccine, Strom said.
The Institute of Medicine panel’s focus on compensation issues echoed repeated calls from labor unions and others for the establishment of a federal compensation fund to reimburse health-care workers who become ill because of the vaccine.
On Thursday, the Service Employees International Union and the American Nurses Assn. called on Bush to delay the start of the vaccination plan until the compensation issues had been resolved. Without such action, union leaders said, they would not be able to support the plan.
And in a letter sent to the president Friday, eight Democratic lawmakers called on Bush to work with Congress to create a no-fault compensation fund for those injured by the smallpox vaccine. Signers of the letter, which called the administration’s refusal to support such a fund “manifestly unjust,” included House Minority Leader Nancy Pelosi (D-San Francisco) and Rep. Henry A. Waxman (D-Los Angeles).
But CDC officials said they were committed to starting the program as soon as possible.
“We are certainly not going to delay this program because of [workers’] compensation issues,” Gerberding said. “This is a high priority and we intend to make this program happen on time.”
Asked why the CDC appeared to be “rushing” the program, Gerberding said, “The president’s decision was based on the fact that we need urgent and efficient action because we live in a dangerous world. We must be prepared.”
On Wednesday, Gerberding told The Times that, because there was no imminent threat of a bioterrorism attack using smallpox, the CDC was emphasizing safety over speed.
In response to other recommendations from the Institute of Medicine committee, Gerberding emphasized the many steps the CDC had taken to prepare for the vaccination program.
She cited, among other things, the “countless hours” it had spent training public-health workers, including those who will train others to administer the vaccine; the many sources of information about the program it had developed, including Web sites, a CD-ROM, information sheets in 14 languages and hotlines in Spanish and English, and the establishment of a board of experts to evaluate the program as it progresses.
The first phase of the program calls for the vaccination of up to 450,000 front-line health-care workers who would be among the first to treat patients infected with smallpox. A second phase would vaccinate up to 7 million additional health-care workers and 3 million police, fire and emergency personnel.
The Institute of Medicine committee strongly recommended a pause between the phases so lessons learned from the first part of the program could be incorporated into the second.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.