Enough Flu Vaccine Will Be Available This Year, Experts Say
- Share via
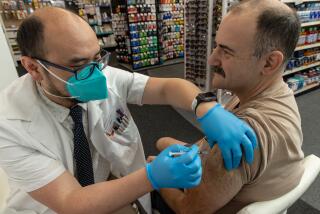
The line for flu shots stretched out the door of an Alhambra drugstore Tuesday morning -- a scene reminiscent of a year ago, when Chiron Corp. scrapped its entire production, triggering a scramble for vaccine.
But this year will be different, industry experts say: Supplies should be adequate for the crowds turning out at clinics and doctor’s offices this flu season.
Although Chiron is struggling to return to the market -- and may not succeed this year -- another manufacturer, GlaxoSmithKline, has entered the U.S. flu vaccine business and plans to deliver 8 million doses.
Most of the people waiting for shots outside the Sav-on Drugs store on East Valley Boulevard were elderly, representing the first wave of vaccine customers. The national Centers for Disease Control and Prevention has taken the cautionary step of limiting the first round of shots to people with the highest risk of developing complications from the flu, including babies, people with chronic diseases and healthcare workers. Public health officials have urged others to wait until Oct. 24 to obtain their flu shots.
The restrictions are expected to prevent the spot shortages and price gouging that plagued last year’s flu season. But some vaccine experts worry that a second year of rationing may have long-term effects on an already fragile market.
Drug industry consultant David Webster predicted that overall demand would fall because rationing would discourage people from getting shots. Last year, 4 million doses were thrown out because no one wanted them by the time they were made available to all.
Webster said about 60 million doses would be used this flu season, barring a severe influenza outbreak. If he’s right, manufacturers -- who are expected to produce more than 70 million doses this year -- would be forced to throw out vaccine once again.
“It could take $200 million out of the market and reduce manufacturers’ incentives to increase [future] production,” Webster said. Chiron, in particular, would suffer if it was unable to distribute its vaccine by Thanksgiving, when demand for shots typically plummets, Webster said.
Investors seem optimistic that the company will succeed: Its shares, which fell below $30 after last year’s vaccine debacle, have nearly recovered and closed Tuesday down 33 cents at $43.53.
Demand for flu vaccine is difficult to predict, as it largely depends on the timing and severity of the flu itself. Two years ago, more than 80 million doses were sold because the flu hit early and hard, sparking demand. By contrast, 57 million of 61 million doses were used during last year’s mild season, despite unusually tight supplies.
Carolyn Bridges, a medical epidemiologist with the CDC, said 50 million doses should be available during October, enough to cover anticipated demand of 42 million doses from people in high-risk groups.
She said it was too soon to predict the severity of the coming flu season, which typically peaks in the winter and runs through March.
Bridges said the CDC, in setting vaccination guidelines, tried to balance the needs of high-risk groups against concerns about wasted vaccine. Last year, such seniors as Magdalene Wong, 66, of Alhambra, weren’t able to get shots. Wong smiled Tuesday as she left the Sav-on store, rolling down her sleeve.
“I don’t want to get sick,” she said.
Exactly one year ago today, the anticipated U.S. vaccine supply was cut in half after British authorities found bacteria in Chiron’s vaccine, forcing the shutdown of the company’s Liverpool factory. Since then, the Emeryville, Calif.-based company has struggled to turn around the troubled plant. Chiron received permission to reopen the factory in March, but the company has not obtained regulatory clearance to ship vaccine to the U.S. The company hopes to deliver as many as 26 million doses by late fall.
Chiron’s problems focused attention on the fragility of the nation’s vaccine supply, with its dependence on a handful of companies. Last season, just Chiron and French-based Sanofi Pasteur made flu shots for the United States. GlaxoSmithKline, a British company, received permission in August to sell vaccine this season.
Most of the vaccine arriving this year is from Sanofi, which expects to supply 60 million doses. In addition to 8 million doses promised by Glaxo, MedImmune Inc. of Gaithersburg, Md., will provide 3 million doses of a nasal-spray vaccine, although the drug has not been approved for babies or the elderly.
Excluding any contribution from Chiron, 16% more vaccine should be available this year compared with a year ago. “We are much better prepared,” said Dr. William Schaffner of Vanderbilt University’s medical school.
In some respects, conditions also are better for manufacturers. Medicare has increased its reimbursement to doctors for giving flu shots and also created financial incentives for nursing homes to vaccinate patients.
The average wholesale price of a flu shot has risen to $10 from $8.50 last year and from $2 in the mid-1990s, a period that saw several manufacturers leave the business. Demand grew through the last decade, until last year. Now some worry that the trend could reverse itself.
Indeed, some large employers, stung by last year’s shortage, have decided against offering flu shots to employees this season, said Steve Wright of Maxim Health Systems, a company that runs corporate flu-shot programs.
More to Read
Inside the business of entertainment
The Wide Shot brings you news, analysis and insights on everything from streaming wars to production — and what it all means for the future.
You may occasionally receive promotional content from the Los Angeles Times.