Bausch failed to report eye infections, FDA says
- Share via
WASHINGTON — Bausch & Lomb Inc. failed to formally report nearly three dozen foreign cases of fungal eye infections later linked to one of its contact lens solutions, according to a federal warning letter.
Bausch & Lomb didn’t submit the 35 serious injury reports by April 7, as required by law, after Singapore health officials had alerted the Rochester, N.Y., company, the Food and Drug Administration said in the letter. The reporting failure occurred after the company had halted sales of its ReNu with MoistureLoc solution in Singapore.
It also came after the FDA had begun an investigation of the Greenville, S.C., manufacturing plant where Bausch & Lomb made the now-withdrawn MoistureLoc solution.
Bausch suspended sales of MoistureLoc in Singapore and Hong Kong in February and U.S. sales in April. It initiated a global recall in May.
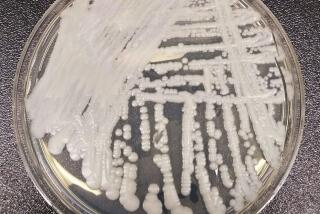
The company later acknowledged that the brand of solution was the potential “root cause” of increased risk of the fungal infection, called Fusarium keratitis.
Health officials reported several hundred cases of Fusarium keratitis in parts of Asia and the United States.
The FDA warning letter refers in part to the inadequacy of a previous company response to FDA concerns about its reporting of the Singapore cases.
In a statement, Bausch said it had completed more than two-thirds of the corrective actions it pledged in response to the FDA’s concerns. The issues stem from the March-to-May inspections of the South Carolina plant.
More to Read
Inside the business of entertainment
The Wide Shot brings you news, analysis and insights on everything from streaming wars to production — and what it all means for the future.
You may occasionally receive promotional content from the Los Angeles Times.