With each beat, a reprieve
- Share via
The first fully implantable artificial heart was approved last week -- a baby step forward in what has been a controversial 30-year dream of some researchers.
For now, the benefits of the device, approved by the Food and Drug Administration for dying heart failure patients who are ineligible for a transplant, outpace the risks by only a slim margin. Even doctors who have experimentally implanted the device see it as a hard sell to surgeons and the few patients to whom it might give some comfortable months of reprieve.
Only about 4,000 patients of the estimated 5 million Americans with heart failure are sick enough to benefit from the heart. Fewer -- 25 to 50 -- are expected to be implanted with it each year.
But slim as the benefits are, the new device, named the AbioCor, reopens a door that was all but slammed shut by the agonizing last days of Barney Clark in 1982. Clark, a retired dentist, suffered multiple strokes before dying as he lay tethered for 112 days to the first artificial heart, the Jarvik-7. Researchers, including inventor Dr. Robert Jarvik, later said they realized that “just being alive is not good enough.”
The AbioCor is very different from the Jarvik. It is much quieter, infinitely tinier and fully enclosed in the body. Patients can be away from an external power source for up to an hour -- enough time to shower, go for a walk or sit down to dinner. Even when the batteries are recharging, they can sit comfortably.
Yet it will still take considerable fine-tuning before this or another artificial heart is an option for patients who have more than a scant month to live. Biomedical engineers will have to solve the tendency of blood to clot when exposed to artificial materials. And while infection is less of a problem for a fully implanted device with no external tubes or wires, the AbioCor still wears out after several months.
For patients so sick, a second 15-hour surgery to replace it is not an option.
The AbioCor, designed by Abiomed Inc., of Danvers, Mass., is distinct from other “assist” devices that are implanted in the body to help the heart. It is a total replacement.
In a procedure that would make poets and romantics cringe, the patient’s beating heart is removed, then replaced not with living tissue but with a two-chambered, titanium and plastic structure that is attached by a special cuff to the remains of the left and right atria.
A battery, recharged across the skin by an external unit, is implanted too, and a rotary device, powered by electricity, keeps blood circulating.
Since 2001, the heart has been transplanted into 14 male patients, one at UCLA. All had less than a month to live. Twelve were too sick to take blood-thinning drugs, which reduce the risk of clots. They eventually succumbed to strokes.
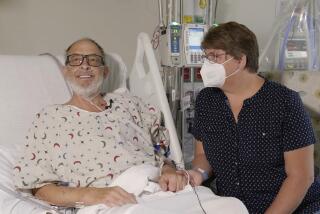
The two men who could take drugs did the best. One of them, Tom Christerson, of Central City, Ky., lived for 17 months, 10 of those in his own home.
“For about five years before the implant ... he could barely walk from his chair to the dining room table, and when he got there, you’d have thought he ran a marathon,” says his daughter, Patti Pryor.
Christerson wasn’t eligible for a heart transplant because of health problems. Visiting a heart surgeon, he saw a prototype of the AbioCor on the physician’s desk. “He said, ‘I’ll try anything but that,’ ” Pryor says.
But with doctors predicting mere days before death, he reconsidered.
He spent six months in the hospital, then another month in a hotel near the hospital before going home. He suffered minor setbacks but always felt comfortable and began feasting on foods he hadn’t been permitted in years: lobster tail, crab legs.
“He stopped by the barbershop every morning. He had coffee with his buddies,” Pryor says. “He and Mom celebrated a 55th wedding anniversary. He was able to hold his first grandbaby.”
The personal reward of a few more months of quality life is inarguable. Not so the public health costs. In time, expenses will decrease, but the device currently costs $250,000 per patient -- with another $300,000 or so in surgical and medical expenses.
Some medical ethicists question the wisdom of this. “With end-stage heart failure and multiple complications, are you going to spend $300,00 to 500,000 as a society to keep them in bed, with multiple problems? Is that humanitarian?” says Arthur Caplan, director of the Center of Bioethics at the University of Pennsylvania.
Michael Minogue, Abiomed’s chief executive, says the cost of the device must be compared with that of treating mortally ill heart failure patients, who can end their lives in long, painful hospital stays, accumulating costs rivaling the AbioCor’s.
Abiomed has already tweaked its device after noticing problems. “With the first five patients, we saw that the cuff was rubbing against atrial tissue,” Minogue says. That increased the risk of clot formation and stroke. The heart was re-engineered, reducing but not eliminating the problem. The next nine patients lived longer.
The AbioCor was designed to last up to 18 months. The company is working on a next-generation replacement heart that they hope will have longer-lasting battery life and components that will keep it pumping five years or longer. They also hope to make it 30% smaller, allowing it to be implanted in women and smaller men. (The AbioCor can be used primarily in men with a large chest cavity.)
Abiomed and other companies are experimenting with -- and debating the relative merits of -- continuous flow pumps, in which the blood moves smoothly through the body, versus pulsating flow, in which the blood is delivered in pulses that mimic the beat of a living heart.
But the future may lie in simpler rotary devices that are suspended in blood with no bearings. “Those could last 10 to 20 years,” says Timothy Baldwin, a program director at the National Heart, Lung and Blood Institute.
Scientists are also experimenting with materials aimed at reducing infections and blood clots, the main complications that arise from placing foreign devices into the body.
“With the advent of nanotechnology, the hope is we can engineer surfaces at the cellular level,” Baldwin says. This would enable engineers to etch the insides of their devices or coat them with materials in a way that encourages blood vessel cells -- endothelial cells -- to better cover the surfaces, making clot-formation less likely.
The NHLBI is also funding research aimed at reducing the size of devices, to help children with congenital heart defects.
Such improvements could expand the field’s promise well beyond what was achieved by last week’s approval of the AbioCor.
“This approval is giving the field a needed boost,” says Dr. Robert Dowling of the University of Louisville School of Medicine, Christerson’s surgeon. Later this month, he’ll begin testing the second generation of the AbioCor device in a cow. “It’s 30% smaller,” he says, “and significantly more durable.”
Times Staff Writer Hilary MacGregor contributed to this report.
susan.brink@latimes.com; hilary.macgregor@latimes.com