Artificial kidney test signals hope
- Share via
Preliminary studies with an experimental artificial kidney that incorporates living cells indicate that it can reduce deaths from acute kidney failure by 50%, Michigan researchers reported Thursday.
The device is meant for short-term use -- up to three days -- to allow a damaged kidney time to recover function.
The artificial kidney “may yield a better treatment for life-threatening acute renal failure, for which a high mortality rate has remained unchanged despite years of advances in conventional therapies,” said Dr. H. David Humes of the University of Michigan, the lead author of a report appearing in the Journal of the American Society of Nephrology.
“They achieved some pretty remarkable results with people . . . who were the sickest of the sick,” said Dr. Leslie Spry of Lincoln Nephrology & Hypertension in Nebraska and a spokesman for the National Kidney Foundation. “This would be a pretty good deal if it gets to be generally accepted.”
Acute kidney failure can arise from trauma, dehydration and a variety of other causes. It affects about 5% of hospitalized patients and a higher percentage of those in intensive care units. The mortality rate is 50% to 70%, even when patients receive the best care available.
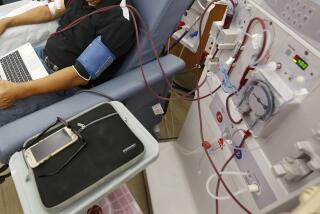
The new artificial kidney, called a renal tubule assist device, or RAD, is a modified form of a cartridge filter that is normally used for continuous dialysis.
Humes and his colleagues devised a technique for plating human kidney cells, called renal proximal tubule cells, on the interior surfaces of the cartridge.
The cells, derived from donor kidneys that are not suitable for transplantation, restore vital electrolytes, salt, glucose and water to the filtered blood. Those components are normally removed during conventional dialysis. The cells also produce immune system molecules, called cytokines, that are important for fighting infections.
Humes and his colleagues studied 58 patients who were critically ill with acute kidney failure at 12 centers around the country. Eighteen of them received conventional continuous dialysis, and 40 received conventional dialysis plus up to 72 hours of treatment with the artificial kidney.
At the end of a month, the team reported, 33% of those receiving RAD therapy had died, compared with 61% of those in the control group. The results were not statistically significant, however, because of the small size of the groups.
After 180 days, 76.5% of the patients receiving conventional therapy had died, compared with 46% of those who used the RAD. That difference was statistically significant.
The team will now undertake a larger trial that is required before the device can be approved for sale.
The trial was sponsored by RenaMed Biologics Inc., a biotechnology company spun off by the University of Michigan to manufacture the device. Humes is a shareholder of the company.
--