RSV season may have already begun, CDC warns, with early activity in the Southeast

- Share via
An increase in the Southeast of respiratory syncytial virus, better known as RSV, may be an early sign of the start of the respiratory virus season.
A health advisory issued by the U.S. Centers for Disease Control and Prevention on Tuesday said there were elevated cases of RSV in parts of the Southeastern U.S. Traditionally, increases of RSV in Florida and elsewhere in the Southeast “have predicted the beginning of RSV season nationally, with increased RSV activity spreading north and west over the following two to three months.”
Federal data show that the rate at which lab specimen tests for RSV are coming back positive, confirming an infection, has risen to just under 5% for the most recent week available, well above the seasonal onset threshold of 3%. In Georgia, officials noted an increase in RSV-associated hospitalizations among children younger than 4.
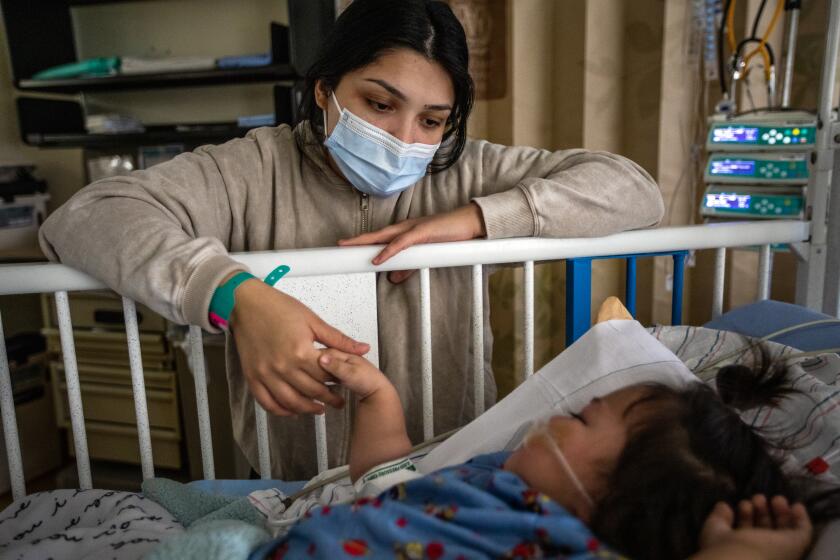
RSV caused significant problems last year, straining children’s hospitals across California and the rest of the nation. RSV causes 100 to 300 deaths annually in the U.S. in children younger than 5 and between 58,000 and 80,000 hospitalizations. Among those 65 and older, RSV causes 6,000 to 10,000 deaths annually and about 60,000 to 160,000 hospitalizations.
The collision of RSV, influenza, COVID-19 and other viruses has strained children’s hospitals across the country, including Loma Linda in the Inland Empire.
For the first time, infants and young children are eligible for monoclonal antibodies, which can be administered early in the season and are designed to protect them from severe RSV should they get infected.
The monoclonal antibody for young children, known by the generic name nirsevimab and the trademarked name Beyfortus — developed by AstraZeneca and Sanofi — was found by federal officials to be safe and efficacious. Data reported by the CDC show that one dose gave infants protection for at least five months, which is the length of an average RSV season, and reduced the risk of severe illness by 80%.
The CDC recommends that all infants 8 months or younger who are entering their first RSV season receive one dose of nirsevimab. Babies 8 to 19 months old who have an increased risk for RSV should get one dose of nirsevimab before or during their second RSV season, the CDC says.
Updated versions and new vaccines for COVID-19, influenza and respiratory syncytial virus will be rolled out this fall.
Also for the first time, vaccines against RSV are available for people age 60 and older. There are two vaccines available, one made by GSK and another from Pfizer. The CDC said healthcare providers should offer a dose of the vaccine “based on shared clinical decision-making between the healthcare provider and the patient,” language that falls short of a full recommendation that everyone in that age group get the vaccine.
“Health care providers and their patients should have a conversation to determine if RSV vaccination will be beneficial,” the CDC said of people over 60. “The decision whether to vaccinate a patient is individually based and informed by discussions between the patient and health care provider.”
Data suggest that both types of RSV vaccines are more than 80% efficacious in preventing RSV-associated lower respiratory tract disease, the CDC said. However, of nearly 40,000 participants in a clinical trial who received either vaccine, six developed inflammatory neurologic events within six weeks of the vaccination, “but it was unclear whether these events were related to [the] RSV vaccination,” the CDC said.
RSV is typically spread through droplets that emerge when an infected person coughs or sneezes.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.