Rotavirus vaccines could reduce deaths in Third World
- Share via
Vaccines that protect against severe disease and death from rotavirus infections in the United States and other developed countries work nearly as well in developing countries and should be widely employed there, researchers report today in two papers in the New England Journal of Medicine.
Health authorities now have “another powerful weapon” to combat the disease, Dr. Mathuram Santosham of Johns Hopkins University wrote in an editorial accompanying the studies. Widespread use of the vaccines could save more than 2 million lives over the next decade, he said.
A third report, however, warns that giving the vaccine to infants who are severely immunocompromised can cause the disease, although the symptoms appear to be mild.
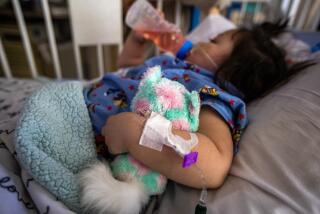
Dehydration caused by severe diarrhea kills more than 500,000 children younger than 5 every year, and rotavirus is the leading cause, accounting for about 40% of all cases. Deaths are relatively few in the United States and other developed countries because of ready access to hospitalization and rehydration therapies, but that is not the case in most of the Third World. A vaccine to prevent the disease could thus make a valuable contribution.
The first vaccine against rotavirus, called RotaShield, was introduced in the United States in 1998 after preliminary studies showed that it was safe and effective. But post-marketing surveillance showed that it caused intussusception -- a severe obstruction of the bowel that can be fatal if not treated -- in about one in every 12,000 children vaccinated and RotaShield was withdrawn the following year.
Two other vaccines against rotavirus have subsequently been approved in the United States, Rotateq by Merck & Co. in 2006 and Rotarix by GlaxoSmithKline Biologicals in 2008. Both are attenuated live virus vaccines and neither appears to produce intussusception when used in young children. The Centers for Disease Control and Prevention recommends that all young children in this country receive one of the vaccines.
A 2008 study showed that early use had delayed onset of the rotavirus season in the United States by three months and reduced severity by half -- even though fewer than half of all children had apparently received even one dose of the three-dose regimen.
But previous studies had shown that live virus vaccines that work in the developed world don’t always work as well in low-income countries. The polio and cholera vaccines, for example, were not as effective, perhaps because of poorer nutrition or higher rates of disease.
Before the World Health Organization would give its approval for donor organizations to start distributing a rotavirus vaccine in the Third World, the agency wanted some proof that it would be effective.
To obtain such proof, a team led by Dr. Nigel A. Cunliffe of the University of Liverpool and Dr. Shabir A. Madhi of the University of the Witwatersrand in Johannesburg, South Africa, organized a clinical trial in South Africa and Malawi. They enrolled more than 4,900 infants and divided into three groups: one group received a dose of placebo at age 6 weeks and doses of Rotarix at ages 10 weeks and 14 weeks, the second received three doses of vaccine and the third received three doses of placebo.
The team reported that the vaccine reduced severe rotavirus disease by 61.2% in the two countries combined. Severe diarrhea occurred in 4.9% of those receiving placebo and 1.9% of those receiving either course of vaccine. The vaccine was less effective in Malawi, a much poorer country than South Africa. Nonetheless, it reduced severe disease there by 49.4%, compared to a 76.9% reduction in South Africa.
Based on preliminary results from the trial, the WHO recommended last June that the vaccines be adopted as part of the childhood vaccination programs in all developing countries.
The second study involved deaths from diarrhea in Mexico, where the Rotarix vaccine was introduced in 2006. The country had already adopted a large program that included improved sanitation, increased use of oral rehydration, breastfeeding and vitamin A supplementation, so it is difficult to completely disentangle cause and effect. Those efforts had not led to a major reduction in disease, however.
A team headed by Dr. Stuart L. Abramson of the Texas Children’s Hospital in Houston found that the average number of diarrheal deaths in the country dropped by more than 40% among children younger than 11 months in 2008, the first year the vaccine was widely used. During the 2009 rotavirus diarrheal season, deaths dropped by more than 65% among children younger than 2.
Dr. Manish Patel of the CDC, a co-author of the study, said: “The reduction in mortality following vaccine introduction points to the importance of immunization against rotavirus as a primary prevention tool in controlling diarrhea not just in Mexico, but around the world.”
A full course of the vaccines can cost from $105 to $160 in the United States, but will be available for less than a dollar in developing countries under international programs. The vaccines do require refrigeration, however, which complicates the process of distribution.
The trials were sponsored by GlaxoSmithKline and the Rotavirus Vaccine Trials Partnership, a collaboration among various public health authorities and funding agencies.
A note of caution was injected by a separate report, also from a team led by Abramson. They identified three infants who developed dehydration and diarrhea after receiving the first or second dose of Rotateq.
All three children were subsequently shown to have severe combined immunodeficiency disease, or SCID, that had not been diagnosed at the time of vaccination. That immune deficiency allowed the attenuated virus to produce disease.
SCID is typically not diagnosed very early in infancy, but Abramson cautioned that physicians should restrain from using the vaccine in infants with a history of persistent infections until their cause is determined.
Rotateq’s label was changed last month to warn against using it in children with SCID.
All three infants recovered without lasting effects from the vaccine.