Ask whether a drug works before worrying about what it costs

- Share via
Last week was supposed to be a turning point for drug price transparency. The Department of Health and Human Services planned to implement a new rule requiring drug commercials on television to disclose the “list price” of the medication being advertised. According to the HHS press release, this would be the most significant single step any administration has ever taken with regard to drug pricing.
But it isn’t going to happen. Three big drug companies, Merck, Eli Lilly and Amgen along with an industry trade group, the Assn. of National Advertisers, filed a successful lawsuit blocking the rule. According to Judge Amit P. Mehta of the United States District Court in the District of Columbia, HHS does not have the authority to mandate price disclosure.
How upset should consumers be? Not very. The HHS argued that the rule would create an incentive to lower prices by shaming companies that charge a lot. But that was wishful thinking. Since when has big pharma responded to shame?
The HHS also argued that the rule would help consumers become “active and well-informed participants in their health care decision-making.” But that was magical thinking. Why? Because how much a drug costs says nothing about what it is worth. To decide if a drug is worth the price, consumers need to know how well it works. That is, they need basic information about a drug’s benefits (what problem does it treat and what is the chance that it will help in some meaningful way) and its harms (how common — and how bad — are the side effects).
Surprisingly, doctors also make a lot of unfounded assumptions about prescription drugs.
For most people, a drug that helps them feel better or live longer would be “worth it,” even at a high price and with side effects. But drugs with only marginal or unimportant benefits, especially if they come with lots of side effects, may not be worth taking at any price.
Unfortunately, good information about drug benefits and harms is hard to find. Direct-to-consumer ads — the most prominent way companies reach consumers — rarely quantify drug benefit. They do not, for example, say something like, “this drug will reduce heart attack risk over the next 10 years from X% to Y%.” Harms are often quantified but are typically enumerated in long lists that people generally ignore (or parody).
Consumers may not even realize how important information about drug benefits and harms is. Many simply assume that advertised drugs work really well. In a national survey, my colleagues and I found that 39% of Americans believed that the Food and Drug Administration approves only “extremely effective” drugs — and 25% believed that the FDA approves only drugs without serious side effects. But neither belief is true. Approval means only that the FDA has concluded that benefits outweigh harms for the drug’s intended use. Many drugs have only a marginal benefit — and all drugs have side effects.
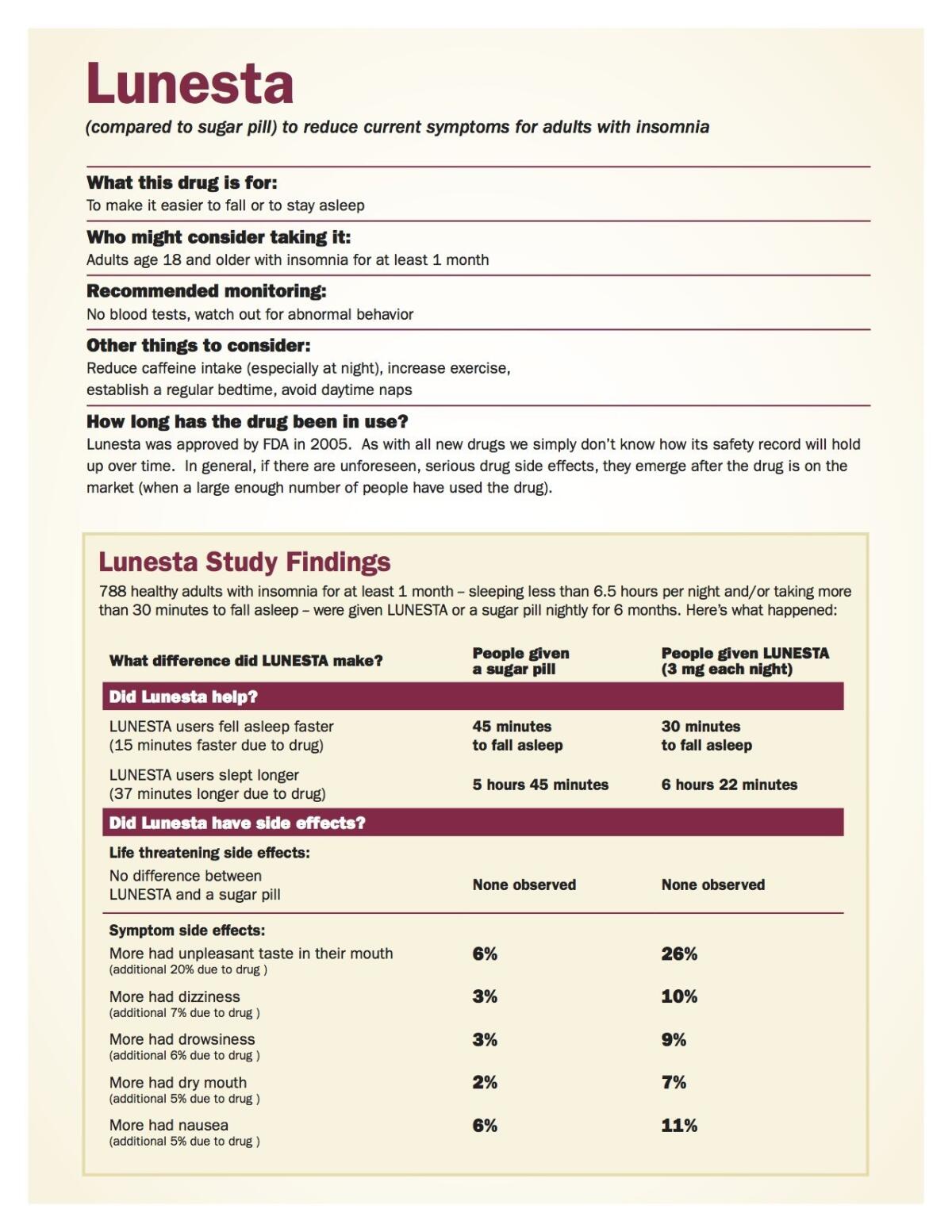
Surprisingly, doctors also make a lot of unfounded assumptions about prescription drugs. In a study of physicians, we found that 70% incorrectly believed that FDA approval required that the drug had a clinically important effect. But many drugs do not. For example, consider Restasis, a heavy advertised prescription-only eye drop to treat chronic dry eye disease. Americans spent more than $8.8 billion on it between 2009 and 2015, including more than $2.9 billion in public funds through Medicare Part D. But the drug was approved only to increase production of tears — it was not approved as having a clinically important effect over inexpensive nonprescription artificial tears on symptoms such as dry, itchy or burning eyes. Better dissemination of the facts might have saved billions of dollars.
Having lost in court last week, the HHS should go back to the drawing board and come up with better ways to help people become wiser consumers of prescription drugs. Judge Mehta’s ruling points out that, unlike pricing information, HHS has the power to regulate drug advertising to ensure direct-to-consumer advertisements are truthful and communicate relevant information concerning a drug’s benefits and risks.
California, which has been so strong in the area of consumer product safety, could take the lead by passing laws to require better communication of drug information to doctors and the public.
Enter the Fray: First takes on the news of the minute »
Getting people access to credible drug is not easy. For years, my colleague Lisa Schwartz and I called on the FDA to require a Drug Fact Box — a brief summary of information about drug benefits and harms derived from the FDA’s own reviews of the evidence — to accompany print or electronic ads. Randomized trials have shown that drug fact boxes improve consumers’ knowledge of drug benefits and side effects and lead to better decisions (i.e., help them choose the objectively better of two drugs). But while the FDA’s own risk communication advisory committee unanimously endorsed this approach, the FDA chose not to act.
A more ambitious solution would be for the HHS to have the FDA commission an app to compare data across drugs (and ideally, non-drug options) used for the same purpose. Unfortunately, such comparative effectiveness data are rarely available, since the FDA does not require new drugs to be tested against those already on the market. If the goal is to promote wiser use of prescription drugs, this needs to change.
In talking up the now failed rule to require prices in drug ads, HHS Secretary Alex Azar said, “You ought to know how much a drug costs and how much it’s going to cost you, long before you get to the pharmacy counter or get the bill in the mail.”
Before worrying about the price, find out what the drug is worth.
Steven Woloshin is a professor of medicine at the Dartmouth Institute for Health Policy and Clinical Practice and director of the Lisa Schwartz Foundation for Truth in Medicine. He is author of “Know Your Chances: Understanding Health Statistics.”
A cure for the common opinion
Get thought-provoking perspectives with our weekly newsletter.
You may occasionally receive promotional content from the Los Angeles Times.



