Pasadena hospital broke the law by not reporting outbreak, health officials say
- Share via
Pasadena’s Huntington Hospital broke state law by not quickly reporting a suspected deadly outbreak last year, according to a letter by city officials.
The hospital released the letter this week, as well as the results of the city’s investigation into the outbreak caused by dirty scopes, which sickened 16 patients, including 11 who died.
City health officials did not investigate the cause of the patients’ deaths, many of whom were seriously ill. The officials noted in the report that only one patient’s death certificate listed as a cause the dangerous drug-resistant bacteria that contaminated the scopes and sickened the patients.
But a lawyer for the families of three patients, including two who died, said that those patients’ medical records show they got infections after procedures with a device known as a duodenoscope.
For example, the death certificate of Thomas Lombardo, a 69-year-old Pasadena teacher, says he died of heart failure and pancreatic cancer.
“His death was almost certainly caused by an infection that could not be treated,” said Pete Kaufman, the lawyer.
“It’s very disturbing,” said Roy Aponno, 52, who survived his infection and is one of Kaufman’s clients. “I think I was one of the lucky ones.”
Eileen Neuwirth, a hospital spokeswoman, noted that Huntington had released the city’s report on Wednesday, immediately after receiving it.
“We take responsibility for the deficiencies outlined in the report and have taken steps to ensure rigorous compliance going forward,” Neuwirth said. “These scopes are an issue facing many hospitals.”
Neuwirth said the hospital can’t comment on ongoing litigation or on specific patients’ cases.
Pasadena officials said in their investigation that Huntington doctors were reviewing suspected drug-resistant Pseudomonas aeruginosa infections in 35 patients before the city’s public health department was alerted to the possible outbreak by county officials on Aug. 19.
State law requires hospitals to report an “occurrence of any unusual disease” or “any outbreaks of disease” within 24 hours to local health officials.
The city’s investigation blamed the scope’s hard-to-clean design, as well as the hospital’s lapses in infection control, for the outbreak.
Pasadena officials said that when they arrived at the hospital Aug. 20, they found visible residue in the machines where the devices were disinfected. They also said the hospital was not properly following cleaning guidelines, including by using canned compressed air from Office Depot to dry the scopes.
Such patient-safety failures can lead to financial penalties levied by the state. The California Public Health Department did not respond to questions Thursday about whether it had fined the hospital or if a state investigation was continuing.
Huntington Hospital had confirmed to The Times in August that three patients were sickened the previous month, but declined to say more about their condition. The hospital later told Olympus Corp., the scope’s manufacturer, that the three patients had died, according to the company’s report to federal regulators.
The city independently confirmed that 16 patients were sickened by the tainted scopes from the first patient case in January 2013 to August 2015.
Pasadena officials had to twice ask the hospital to notify patients who had procedures with the three duodenoscopes that tested positive for the bacteria over those two years and seven months, the city said in its report.
Hospital officials didn’t agree to notify all of those patients until late last month. Before then, doctors had only notified patients who had procedures with the scopes from January 2015 to August 2015.
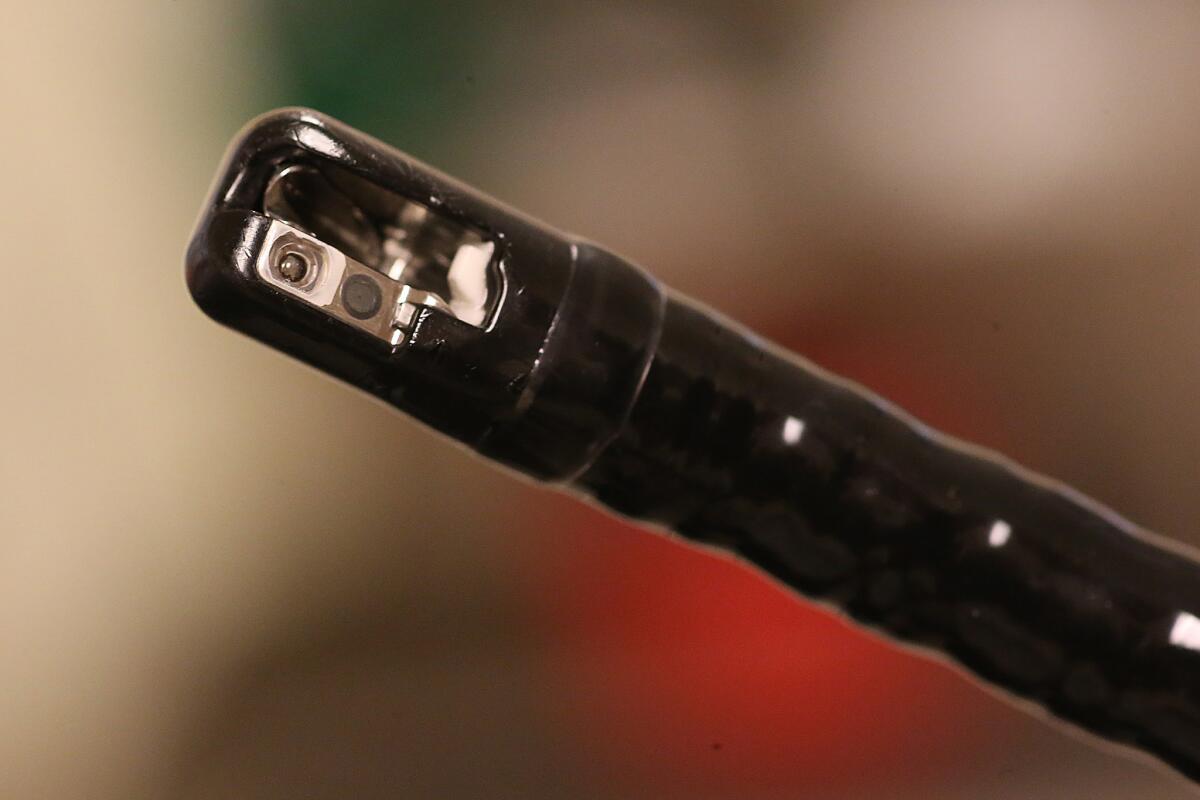
The duodenoscope is a long snake-like tube with a tiny camera on the tip that is inserted through a patient’s mouth and throat to treat cancers and other problems in the upper gastrointestinal tract.
Olympus recalled one of its duodenoscope models in January to replace an internal mechanism that an independent investigator had said in 2012 could allow bacteria to be trapped inside. Huntington was using that model and also an older model that had been believed to be safer.
Aponno, 52, said in an interview Thursday that he had five duodenoscope procedures at Huntington from late 2014 to June 2015 to treat gallstones. He then had surgery that same month and ended up in the hospital for three weeks, in severe pain and on antibiotics and morphine.
He said the doctors were vague about what he was suffering from, asking him at one point if he had traveled to West Africa.
“They were never really straight about what the problem was,” he said. “I asked them, ‘Why can’t I go home? What is the problem?’”
After the outbreak was reported in the news, a Huntington doctor called Aponno and asked how he was doing. The doctor told him, he said, that he was “probably among the patients who got this superbug.”
ALSO
Shares jump on IPO for Soon-Shiong start-up NantHealth
Tribune Publishing renames itself Tronc as its dispute with Gannett continues
Will more start-ups follow Uber in taking cash from sovereign wealth funds?
More to Read
Inside the business of entertainment
The Wide Shot brings you news, analysis and insights on everything from streaming wars to production — and what it all means for the future.
You may occasionally receive promotional content from the Los Angeles Times.











