Pricey generic drugs to get competition from Imprimis
- Share via
Turing Pharmaceuticals sparked nationwide outrage and government investigations by raising the cost of Daraprim, a drug for treating AIDS and cancer, from $13.50 a capsule to $750 last month.
This week, the biomedical company Imprimis Pharmaceuticals of San Diego introduced a competitor to the medication — for $1 a capsule.
Imprimis plans to take the same strategy to undercut other generic drugs sold at far more than their manufacturing cost. Like Daraprim, these medications tend to be used in niche markets that until now haven’t attracted much competition.
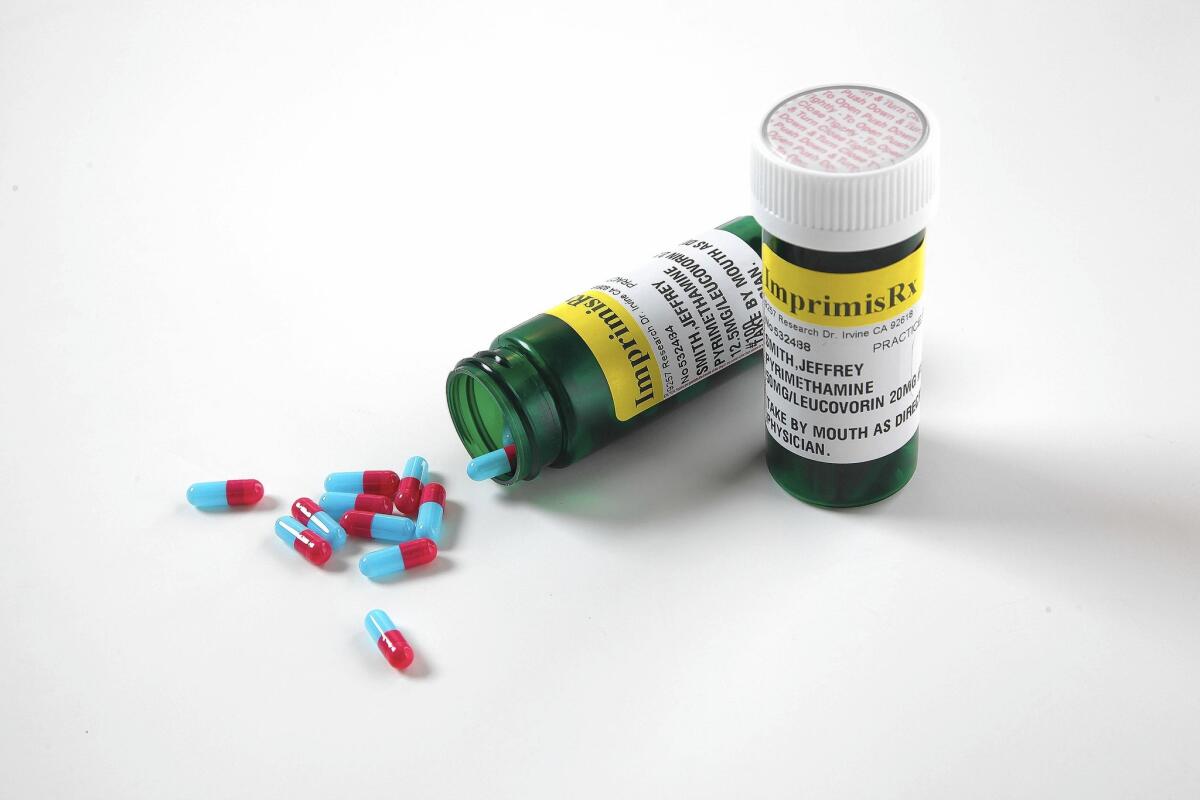
Daraprim is a brand-name formulation of the generic drug pyrimethamine. Turing’s chief executive, Martin Shkreli, has gained notoriety because his company acquired the rights to it — then raised its price 5,000%.
Imprimis’ version combines pyrimethamine with another generic drug. This strategy allows Imprimis to operate as a compounding pharmacy, which doesn’t require approval from the U.S. Food and Drug Administration for its products. The setup spares the company a long FDA review process — and saves it money.
Turing isn’t the first company to suddenly raise the price of a generic drug. Valeant Pharmaceuticals became well-known for the practice. It increased the prices of two heart drugs, Nitropress and Isuprel, by a respective 212% and 525%, immediately after acquiring them.
Valeant also raised the price of the San Diego-originated heartburn drug Zegerid by 550% this year, according to Deutsche Bank.
Valeant’s actions have provoked a storm of opposition. Politicians have joined the fray, including Democratic presidential candidates Hillary Rodham Clinton and Bernie Sanders, the U.S. senator from Vermont.
But Turing took the practice to a new level, Imprimis CEO Mark Baum said. He hopes his company’s response provides a market-based answer to exorbitant drug pricing.
Pharmaceutical companies argue the high prices pay for expensive research. Shkreli says his company’s pricing finances innovative drugs and provides a profit motive to bring existing drugs to market.
The company spends more than half its revenue on research and development, Shkreli wrote on Twitter.
“Please get your facts straight before lumping us in with others,” he wrote.
Turing also said it offers major price discounts for patients in programs such as Medicaid.
However, a company that buys a generic drug didn’t pay for the research and development expenses — and its purchase price is far lower than if the medication was still under patent. That’s why industry groups like Pharmaceutical Research and Manufacturers of America have condemned actions such as Turing’s and Valeant’s.
“There was no justification for it other than profit,” said Joe Panetta, CEO of the San Diego-based life science trade group Biocom.
Turing was able to dramatically raise the price of Daraprim because it was the only seller of pyrimethamine, which is used to treat various infections like the parasite-linked toxoplasmosis. Toxoplasmosis is dangerous for people with weakened immune systems, such as those with HIV and patients undergoing chemotherapy.
Pyrimethamine is generic, meaning that any qualified drug maker could introduce a competitor. But it occupies a niche market, and its previously low price meant that other companies didn’t see a market opportunity.
Turing’s huge price hike provided an opening for Imprimis.
Imprimis said it now offers customized, compounded formulations of pyrimethamine and a type of folic acid in capsules for as low as $99 for a bottle of 100 capsules.
Baum noted that there’s a limitation: The formulation can be sold only through a doctor’s prescription made out to a specific individual, not as an over-the-counter product. Although Imprimis’ combo drug isn’t FDA-approved, the drug’s ingredients have been cleared by the FDA, and the company’s compounding operations have been cleared by the agency.
Filing for FDA approval of the compound itself would take years and millions of dollars, Baum said.
Although complaints about high-priced drugs are long-standing, what’s new is that the prices of formerly inexpensive drugs are now being raised significantly — making them unaffordable for some patients, said Dr. Sherry Franklin, a pediatric endocrinologist in Solana Beach.
For example, she said the rising cost of doxycycline, used to treat acne, makes it impractical for that purpose. The price has reportedly risen in recent years by 600% or more.
“The bottom line is the price of drugs in the United States of America is ridiculous,” Franklin said. “I think it’s reached a point where we can’t continue to do this.”
Fikes writes for the San Diego Union-Tribune.
bradley.fikes@sduniontribune.com
More to Read
Inside the business of entertainment
The Wide Shot brings you news, analysis and insights on everything from streaming wars to production — and what it all means for the future.
You may occasionally receive promotional content from the Los Angeles Times.








