Why it took years for the FDA to warn about infections tied to medical scopes
- Share via
An outbreak at a Pennsylvania hospital in late 2012 should have been an early warning that a reusable medical scope was spreading deadly infections and nearly impossible to disinfect.
But staff at the federal Food and Drug Administration lost the report, one of multiple missteps that allowed doctors and hospitals to continue using the scope for three more years even as dozens of patients were sickened.
The missing paperwork, revealed in a recent Senate inquiry, underscores the serious shortcomings in the antiquated national database used to monitor the safety of medical devices, which even the FDA has long admitted is flawed.
But the fix called for by the Senate investigators — the speedy implementation of a new system already a decade in the making — has hit a roadblock put up by two powerful opponents who say an essential part of the safety upgrade will cost too much.
Patients may now have to wait another decade for the new system, a delay that could lead to more patient deaths.
“We need to build a better system to find these problems more quickly,” said Dr. Josh Rising, director of healthcare programs at the Pew Charitable Trusts.
Further postponement, he said, “could compromise the safety of millions of Americans.”
The device known as a duodenoscope is only the most recent example of a risky medical device that was used in tens of thousands of patients before regulators finally pinpointed a deadly problem in its design. Regulators did not warn hospitals about its risks until after The Times reported an outbreak at UCLA that killed three patients.
Two days after the Senate report was released last month, Olympus Corp., the scope’s leading maker, said it would recall and redesign the device.
Other devices recalled in recent years include metal-on-metal hip replacements, vaginal mesh used in surgeries and lead wires in heart defibrillators.
To more quickly find the problems, Congress passed a law in 2007 requiring that each device get a unique number, like a bar code, that would correspond to the specific brand and model. The number would be recorded in patient records and medical claims.
Experts say the new system will enable regulators or doctors to quickly check large insurance databases to discover how frequently a device is malfunctioning.
But the law included no deadlines. And it took the FDA until 2012 to finalize rules requiring manufacturers to stamp the “unique device identifiers” on their products.
Manufacturers began putting numbers on some devices — those considered most critical — in 2014. Duodenoscope manufacturers must begin printing the numbers on their products in September.
But with the system moving closer to completion, a trade group of the nation’s hospitals and the Obama administration’s Medicare directors raised objections to a key part of the plan: adding a line to the standard insurance claim form so that the device numbers can be recorded.
Join the conversation on Facebook >>
They say reprogramming computers to process the device numbers would be an expensive task.
“Hospital internal billing systems do not currently exist to accommodate” the device number, George Arges of the American Hospital Assn. testified at a hearing in 2014. “Who will pay providers for undertaking such changes?”
Federal Medicare directors have echoed the hospitals’ concerns.
In a letter last year to two senators, Marilyn Tavenner, then the administrator of the Centers for Medicare and Medicaid Services, or CMS, wrote that including the device number on the claims “would entail significant technological challenges, costs and risks to normal claims processing for Medicare and other payers.”
Without device identifiers on claim forms, there would be few ways to track the devices and their impact on patients, greatly limiting the system’s usefulness.
“It’s as if each package that FedEx shipped had an identification number, but you couldn’t tell which one was connected to your package,” explained Peter Orszag, a Citigroup executive and former director of the Office of Management and Budget in the Obama administration, in a column last year.
Supporters of the new system — including AARP and the insurer Aetna — estimate that the nation’s health system would eventually save money overall by more quickly removing devices that cause injuries.
The scope-related outbreaks, for example, resulted in many patients staying in the hospital for weeks or months as doctors tried to fight their infections with heavy doses of antibiotics. In some cases, it took hospitals weeks to link the infections to the scopes because they were unaware of similar problems elsewhere. The treatments cost millions of dollars.
Hospital association officials said in an interview that they support the use of the device identifiers. But they said adding the numbers to insurance claims would not be practical.
“It’s just not going to be possible to track every device used in the care of the patient,” said Chantal Worzala, the association’s policy director.
A CMS spokesman said that the agency was working with the FDA to “to explore options that would improve surveillance in a timely and effective manner.”
Sen. Patty Murray (D-Wash.), whose staff led the investigation into the outbreaks, last month called on Congress to require the device number to be included in claims.
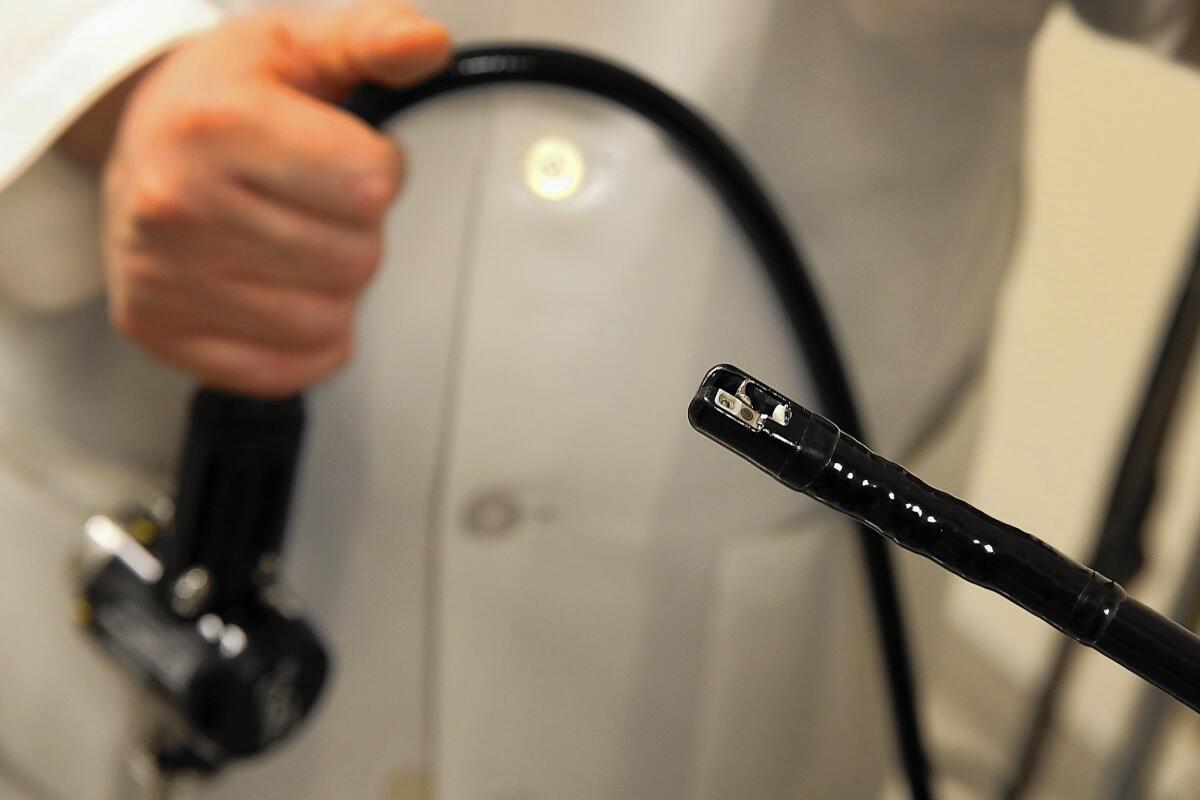
The duodenoscope is a long snakelike tube with a tiny camera on its tip. It is used more than 600,000 times a year to treat cancer and other problems in the digestive tract.
Not only is the FDA’s existing database of device injuries and deaths difficult to use, but it relies on hospitals and manufacturers to report problems they are often reluctant to disclose because of the possibility of litigation.
In their Jan. 13 report, Senate investigators found that not one of 16 or more American hospitals with outbreaks linked to scopes properly filed the federal report required when a medical device kills or injures a patient.
Some of the hospitals eventually reported the outbreaks to the manufacturer. But manufacturers are allowed to rewrite those injury reports before sending them to the FDA’s database.
The investigators found that Olympus had submitted incomplete and misleading reports. Some of Olympus’ reports understated the number of patients infected, the investigators said. And in some reports, the company tried to blame the hospitals for not properly cleaning the scope.
Olympus did not respond to a request for comment.
By the time the FDA began looking into whether the $40,000 reusable scope was transferring bacteria among patients in September 2013, Olympus and another scope manufacturer had sent five reports of outbreaks to the agency. But when the FDA staff searched the database, they found just one of those reports, according to investigators.
It took another 17 months for the agency to warn hospitals the scope was almost impossible to clean.
By then, at least 195 patients in 16 hospitals across the country had been sickened with lethal bacteria — though the toll is probably far higher, investigators found.
Deborah Kotz, an FDA spokeswoman, said the agency was already working on the report’s recommendations.
“The FDA is committed to establishing a national medical device evaluation system that leverages real-world evidence to help us more quickly identify safety signals,” Kotz said.
The agency now makes a disclaimer when it uses information from its database to evaluate the safety of a device.
Recently it noted on its website that data extracted from the system may be “incomplete, inaccurate, untimely, unverified, or biased.”
Twitter: @melodypetersen
Times staff writer Kim Christensen contributed to this report.
SIGN UP for the free California Inc. business newsletter >>
MORE BUSINESS COVERAGE
Salinas hopes to turn farm workers’ children into computer scientists
Oil’s latest slump takes a heavy toll on Bakersfield
Second-half Super Bowl ads are filled with juke box heroes
More to Read
Inside the business of entertainment
The Wide Shot brings you news, analysis and insights on everything from streaming wars to production — and what it all means for the future.
You may occasionally receive promotional content from the Los Angeles Times.











