Study of shingles vaccine supports wider use
- Share via
A study of more than 300,000 elderly patients showed that the underutilized herpes zoster vaccine reduced the incidence of painful shingles outbreaks by 55%, even in the oldest populations, researchers reported Tuesday.
The results suggest that the vaccine, which was introduced in 2006 and now reaches only about 11% of the elderly population nationwide, should be used much more widely, experts said.
Shingles is a painful rash brought on by the varicella zoster virus, which also causes chickenpox. Once chickenpox subsides, the virus can remain hidden in a person’s body for decades before erupting once more.
In addition to pain, a rash can cause vision loss if it spreads to the eyes. The most severe complication is post-herpetic neuralgia, in which the virus causes irritation and inflammation inside nerves. That is a persistent, painful condition that can go on for years and is “extremely challenging to control,” said Dr. Bruce Hirsch, an infectious diseases specialist at North Shore University Hospital in Manhasset, N.Y., who was not involved in the research. “Some patients have even been driven to suicide.”
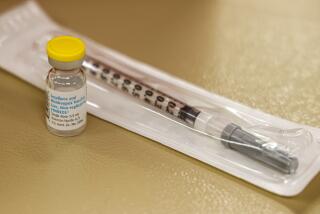
The herpes zoster vaccine, sold as Zostavax by Merck and Co., was introduced in an attempt to prevent such complications.
Epidemiologist Hung Fu Tseng of Kaiser Permanente’s department of research and evaluation in Pasadena led a team that checked the effectiveness of the vaccine among the company’s members in Southern California.
“We thought we might want to take a look at how the vaccine really performed in a real-world situation,” Tseng said.
Tseng and his colleagues examined the records of 75,761 vaccinated and 227,283 unvaccinated men and women over 60 from 2007 to 2009. Among those who were not vaccinated, there were 13 cases of shingles for every 1,000 person-years (a measure of the total number of years that all study participants were followed), but only 6.4 cases among those who were vaccinated.
That means one case of shingles was prevented for every 71 people vaccinated, the team reported in the Journal of the American Medical Assn.
“This helps our comfort level, knowing that results in the real world …confirm what was done in the clinical trial,” Hirsch said.
The original clinical trial that led to the vaccine’s approval found that it was not as effective in people over 75, but Tseng’s results found that age did not make any difference. “That’s still a mystery to us,” he said. “It will need more study.”
Currently, the Centers for Disease Control and Prevention recommends the vaccine for all people over 60 unless they are immunosuppressed, have leukemia or lymphoma, are HIV-positive or are allergic to any of the components of the vaccine. The agency is thought to be considering expanding the recommendation to begin at age 50.
Kaiser offers the one-time vaccine to its elderly members who want it, as do some other health plans and insurers. For those whose insurance does not cover it, however, the price is steep — about $200. But it may be worth it, Tseng said: “Shingles is not something you want to have.”
The study was funded by Kaiser Permanente.