Psychedelic drugs change brain cells in ways that could help fight depression, addiction and more

- Share via
Psychedelic drugs’ mind-expanding properties may be rooted in their ability to prompt neurons to branch out and create new connections with other brain cells, new research has found.
This discovery may explain why psychedelic drugs appear to be a valuable treatment for a wide range of psychiatric diseases, scientists said.
In test tubes as well as in rats and flies, psychedelic drugs as diverse as LSD, ecstasy, psilocybin and ketamine all share this knack for promoting neural “plasticity,” the ability to forge new connections (called neurites) among brain cells. In particular, the drugs appeared to fuel the growth of dendritic spines and axons, the appendages that brain cells of all sorts use to reach out in the darkness and create connections, or synapses, with other brain cells.
The study, led by UC Davis chemist David E. Olson, was published Tuesday in the journal Cell Reports.
The discovery of this neurite-promoting property could shed light on why these chemically distinct drugs all appear helpful in treating depression, anxiety, addiction and post-traumatic stress disorder, or PTSD, Olson said.
It also may point the way to the creation of compounds that mimic the plasticity-promoting properties of these drugs without inducing the potentially dangerous hallucinations, out-of-body experiences and full-on euphoria.
“Chemists are very clever and can modify structures to enhance certain properties and take away others,” Olson said. “We’re attempting to do that with these compounds,” and having a wider variety of compounds to work with makes the prospect of success more likely, he said.
In humans, depression, anxiety disorders and addiction affect many of the same neural circuits. And researchers have consistently found that in people affected by these disorders, the neurons of the prefrontal cortex — the seat of what’s called executive function — have typically lost the dense connections to other brain cells that make a healthy brain hum.
Researchers have long known that ketamine, a sedative that produces a trance-like state and is widely used as a party drug, promotes the growth of neurites in the prefrontal cortex. When infused at low doses into patients with life-threatening depression, ketamine has been shown to be a powerful and fast-acting antidepressant. More recently, it has demonstrated promise as a treatment for PTSD and heroin addiction.
In August, the U.S. Food and Drug Administration designated MDMA (a shorthand for the chemical name of ecstasy, an amphetamine) a potential “breakthrough therapy” as a treatment for PTSD, a status that will speed testing. An FDA-approved clinical trial of the ergoline LSD as an adjunct to psychotherapy in 2014 found it had powerful anti-anxiety effects in those facing life-threatening illness, and more trials are planned. Psilocybin, an ingredient in magic mushrooms, is from the chemical family of tryptamines and has been found highly effective in the treatment of patients suffering such “existential anxiety.”
But researchers still don’t know exactly how ketamine or these other classes of psychedelic drugs work to banish suicidal depression and related ailments.
That is what Olson and his team set out to explore.
In lab dishes, they found that LSD and chemical compounds that mimicked psilocybin and ecstasy increased the complexes of branches sent forth by rats’ cortical neurons on a par with ketamine.
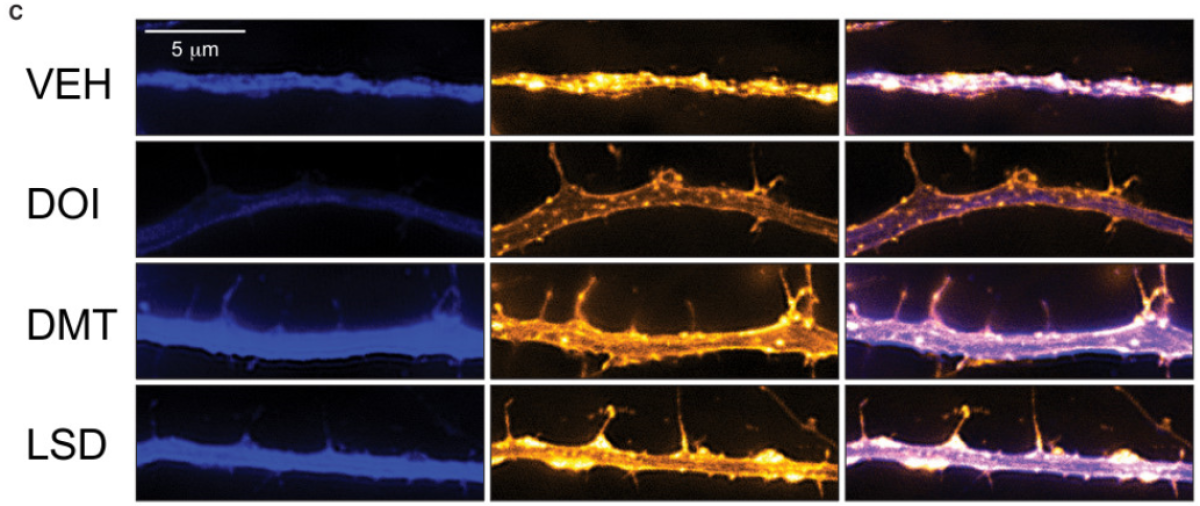
The effect was “remarkably potent” in LSD, even at very low doses, Olson said. This might shed light on the increasingly popular practice of “microdosing,” in which people take very small doses of LSD or psilocybin in a bid to be happier and more creative.
In lab rats, they found that their chemical mimic of psilocybin had the same powerful effect — and did so by a mechanism thought to be at work in ketamine.
Both psilocybin and ketamine, their findings suggested, appear to promote the growth of axons and dendrites by setting off a cascade of neurochemical events normally governed by the mTOR gene, which in turn triggers production of proteins necessary for the formation of new synapses.
In rats, the psilocybin treatment produced results that persisted for hours after the compound had been cleared from the body. That suggests that in humans, the drug’s neuroplasticity-promoting effects could last well after it has worn off.
“Prior to this study, there was only one player in town and that was ketamine,” Olson said. By demonstrating that drugs with very different chemical structures act like ketamine, “this opens up some new doors.”
The more chemical starting points pharmaceutical researchers have to work with, he added, “the better chance we have of developing something safer.”
MORE IN SCIENCE
Suicides have increased by more than 30% since 1999 in half the states, CDC says




