FDA Blamed for Holding Up Rapid AIDS Tests
- Share via
WASHINGTON — Officials at the federal Centers for Disease Control and Prevention, in a rare show of discord between two major health agencies, are complaining that the Food and Drug Administration is holding off the market new AIDS tests that give quick results and could reduce new infections.
Fearing the social stigma still attached to AIDS, 25% of those who get AIDS tests--about 10,000 people each year--never come back for the results, the Atlanta-based agency estimates. Those who are infected go untreated, spreading the virus unknowingly.
The new tests, which give results in minutes rather than days or weeks, could change that, allowing treatment with powerful new drugs almost immediately.
Several new, so-called rapid HIV tests are moving toward FDA approval. But officials at the CDC who deal with AIDS testing and prevention think that they could move even faster.
“I think the FDA is being overcautious, which is wasting time and costing lives,” said Dr. Bernard Branson, one of those officials. He is a medical epidemiologist at CDC who has studied the effect that rapid tests would have on the AIDS epidemic.
Problem Exacerbated for Health Officials
Whatever the reason some people fail to return for test results--denial, fear, inconvenience--the situation poses a vexing problem for the public health community, which sees early identification of infection as one of its most effective weapons against AIDS.
As a result, the FDA is taking heat not just from its sister public health agency but from others in the AIDS network, including those who work in clinics, and from state and regional public health officials. All are impatient to begin using the new tests.
“The waiting period is one of the cruelest elements of HIV testing,” said Lee Klosinski, director of education for AIDS Project Los Angeles. “It is having to endure two weeks of hell. Because of it, people get lost. It is the best reason for expediting this technology.”
And Julie M. Scofield, executive director of the National Alliance of State and Territorial AIDS Directors, wrote in a recent letter to the FDA: “Early identification of HIV infection is essential. . . . It is critical that rapid testing be made available in the U.S. as soon as possible.”
But Dr. Jay Epstein, director of the FDA’s office of blood research in its center for biologics evaluation and research, defended his agency.
“We understand there is an unmet need,” he said. “We agree it would be good to have more on the market. But it’s not a simple thing. The public needs assurance that the [new tests] will meet standards of accuracy and consistency of manufacturing.”
Epstein insisted that the FDA is taking steps to expedite approval, explaining that rapid test proponents “misunderstand” the regulatory requirements for licensing of the new tests.
To that end, an FDA advisory committee will take up the matter Thursday in an attempt to clarify what is expected in the way of studies--and results.
He added that clinics can apply under special circumstances for permission to use the new tests before they are licensed. “We are trying to show flexibility and cooperate with CDC.”
Accuracy Is Still a Key Requirement
The FDA has asked its Blood Products Advisory Committee to recommend that the rapid tests not have to prove that they are as effective as the test most widely used now in clinics nationwide--known as the ELISA. But the new tests still will have to demonstrate a high level of accuracy.
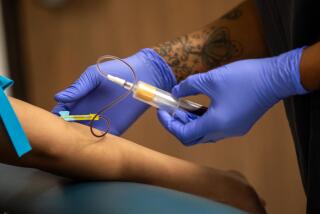
The ELISA test takes a minimum of five hours to work, sometimes as long as overnight. But since the test often is sent to labs--where it is run in batches--results often are not available for days, even weeks.
If rapid tests are approved, several would be needed for the diagnostic system to work well. Currently, ELISA results must be confirmed by a different, more sophisticated test, known as the Western blot.
Once licensed, several rapid tests likely would be used at the same time to statistically validate accuracy. This would protect against the possibility of “false positive” or “false negative” results.
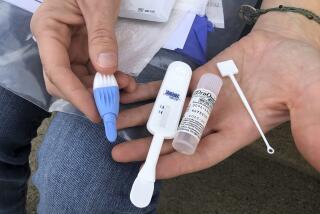
There is only one rapid test on the market. Known as SUDS, it is made by Illinois-based Abbott Laboratories. It works in about 10 minutes, but its accuracy is highly dependent on the level of training of the person who gives it.
New Tests Seen as Attractive Option
The new tests under development are more user-friendly, requiring no equipment and little technical expertise. Some work on whole blood, some work on oral fluids. And they are inexpensive, costing less than $2, which makes them an attractive option, not just for clinics but for hospital delivery and emergency rooms and in developing countries that lack laboratories and other technology, the CDC’s Branson said.
At the Hollywood/Wilshire Health Center and the Gay and Lesbian Health Center in West Hollywood, a major study sponsored by the CDC and Los Angeles County has shown that the rapid tests are accurate.
The first phase of the study involved individuals who already knew their infection status. It compared the known results with those of the rapid tests, which proved accurate.
The study is now looking at these tests in actual practice, that is, conducting them on people who have not been previously tested--then comparing the results to those obtained using current technology.
Thus far, the rapid tests “are all great and extremely useful,” said Apurva Uniyal, coordinator of the study. “I think all of them will prove useful in different settings.”
These settings potentially benefit patients beyond those visiting AIDS clinics. Many pregnant infected women, for instance, have no contact with the health care system until they deliver their babies. If they can be identified as infected--when they come in to give birth--drugs can be given to their babies during and after delivery that greatly reduce chances of the virus being transmitted from mother to child.
Rapid tests could be important in rural clinics, mobile testing units and hospital emergency rooms. The CDC estimates that as many as 12% of those who show up in inner-city urban emergency rooms are infected but do not know it.
CDC researchers, using a mathematical model, have estimated the potential effect of using rapid testing in publicly funded HIV test sites.
Their findings show that in one year alone, nearly 700,000 more people--including more than 8,000 infected individuals--would learn their HIV status if rapid tests were used.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.